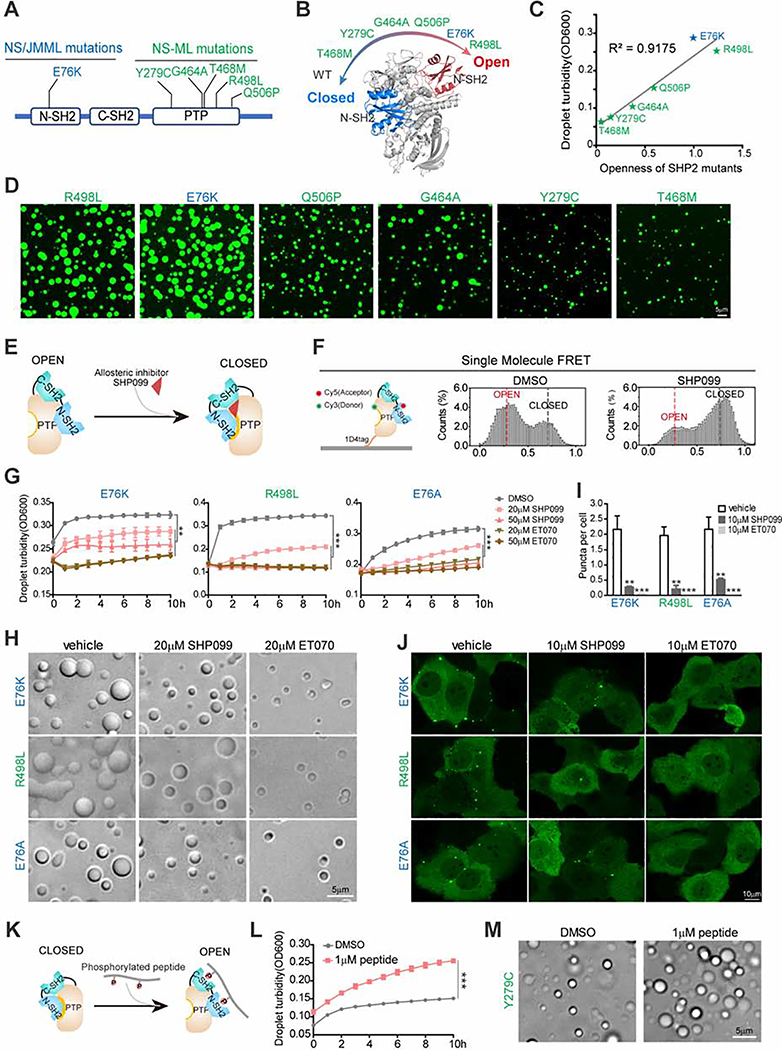
Fig. 4. Open conformation promotes SHP2 LLPS.
(A) Representative disease-associated SHP2 mutants. NS/JMML mutations (blue) and NS-ML (green). (B) A scheme representing the intrinsic propensity for open conformation in SHP2WT and indicated SHP2mut. (C) The correlation between in vitro LLPS capability and openness of SHP2 mutants. (D) Representative images of recombinant indicated SHP2mut-mEGFP protein droplet formation in the presence of 10%(w/v) PEG3350. Scale bar, 5 μm. (E) A scheme illustrating allosteric inhibitor SHP099 locks SHP2 in the closed conformation. (F) Single molecule FRET results show the conformation change of SHP2E76A-87/266 induced by SHP099. See Figure S4A for representative FRET time-trajectories. (G, H) Indicated SHP2mut droplets were treated with SHP099 and ET070. Time course of droplet turbidity OD600 (means ± SEM, N = 3 experiments) **p<0.01; ***p<0.001. (G) and representative images (H). Scale bar, 5 μm. (I, J) KYSE520 cells stably expressing indicated SHP2mut–mEGFP treated with SHP099 or ET070. Quantification of puncta/nuclei (means ± SEM) **p<0.01; ***p<0.001. N=46 cells. (I) and representative images of indicated SHP2mut–mEGFP (J). Scale bar, 10 μm. (K) The cartoon for binding of the bis-P peptide to SHP2. (L) SHP2Y279C proteins were stimulated w/o 1 μM bis-P peptide and the droplet turbidity (means ± SEM, N=2 experiments) was assessed. ***p<0.001. (M) Images of SHP2Y279C droplets treated with DMSO and 1 μM bis-P peptide. Scale bar, 5 μm.