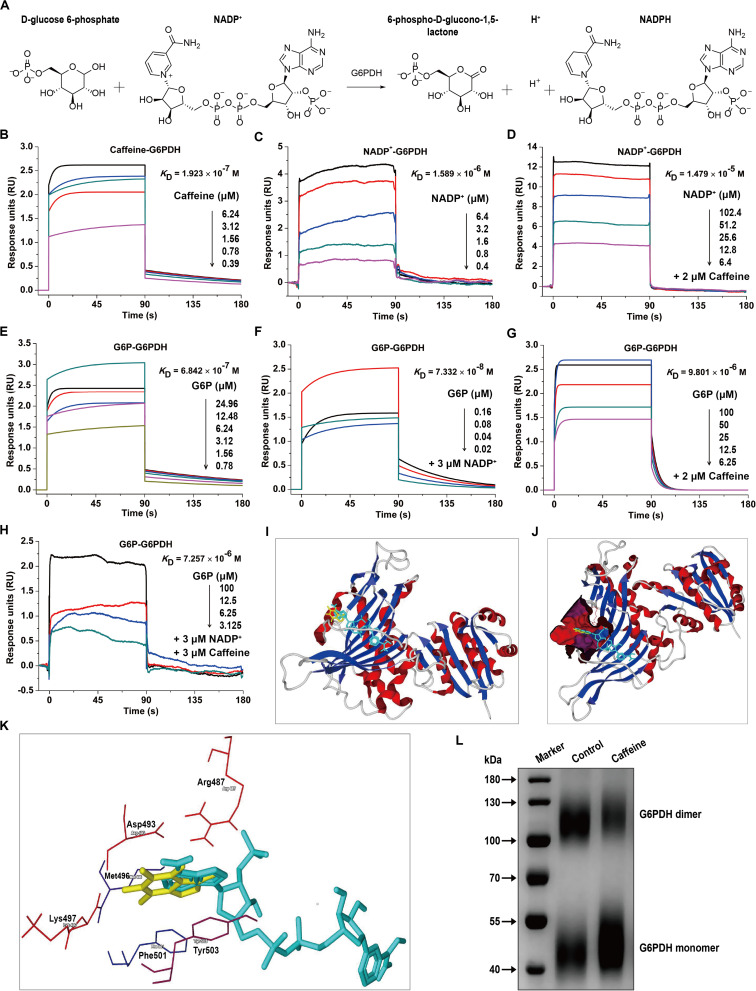
FIGURE 2.
Caffeine directly binds to G6PDH and decreases its coenzyme and substrate binding affinities. (A) The enzymatic reaction process involving G6PDH. (B) Caffeine directly binds to G6PDH, with a binding affinity of KD = 1.923 × 10– 7 M. (C) The coenzyme NADP+ binds to G6PDH, with KD = 1.589 × 10– 6 M. (D) NADP+ binds to G6PDH in the presence of caffeine (2 μM), with KD = 1.479 × 10– 5 M. (E) The substrate G6P binds to G6PDH, with KD = 6.842 × 10– 7 M. (F) G6P binds to G6PDH in the presence of NADP+ (3 μM), with KD = 7.332 × 10– 8 M. (G) G6P binds to G6PDH in the presence of caffeine (2 μM), with KD = 9.801 × 10– 6 M. (H) G6P binds to G6PDH in the presence of NADP+ (3 μM) and caffeine (3 μM), with KD = 7.257 × 10– 6 M. (I–K) The computational binding mode of caffeine to human G6PDH (PDB code: 2BH9) was established using the MVD molecular docking software. (I) Caffeine (yellow) binds to G6PDH (ribbon) at the structural NADP+ (light blue) binding site. (J) Caffeine (yellow) and NADP+ (light blue) are both bound in the same pocket (magenta) of G6PDH (ribbon). (K) The binding pocket for caffeine (yellow) and NADP+ (light blue) is formed by six important residues in G6PDH, shown in sticks: Arg487, Asp493, Met496, Lys497, Phe501, and Tyr503. (L) Caffeine inhibits the formation of dimeric G6PDH, as demonstrated by chemical cross-linking analysis. All SPR experiments were performed using a Biacore S200 instrument. The data displayed here represent one of three independent experiments with similar results.