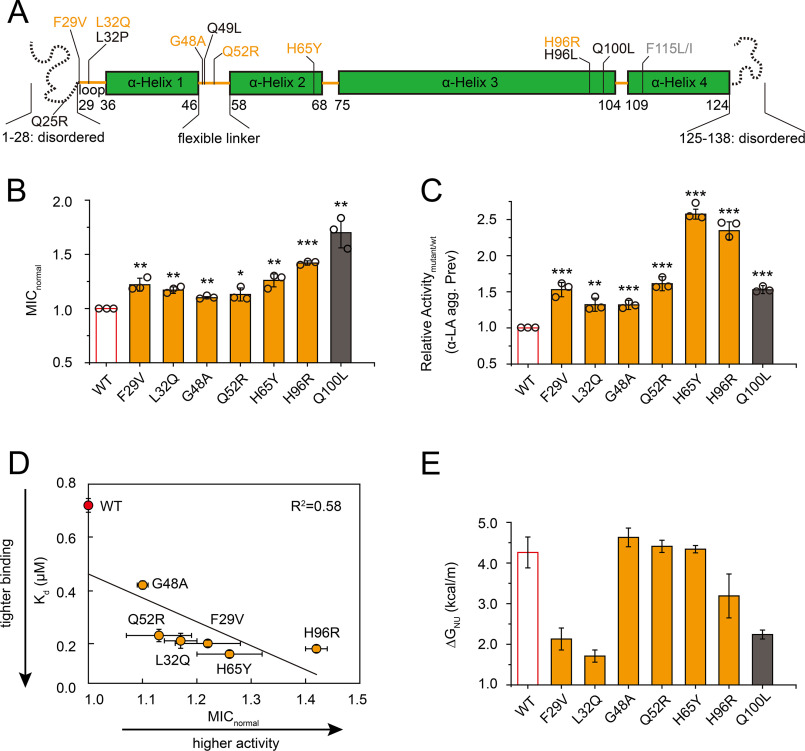
Figure 1.
Characterization of the new gain-of-function variants in the chaperone Spy. A, distribution of the beneficial mutations on Spy's sequence, with the secondary structures annotated. Variants identified in this study (but not in the previous work) are shown in orange, those obtained in both studies are shown in black, and those only isolated in the previous work are shown in gray. B, all newly isolated Spy variants exhibited enhanced in vivo chaperone activities (n = 3, Student's t test). *p < 0.05; **p < 0.01; ***p < 0.001. The variants' in vivo activities are expressed as normalized MIC (MICnormal = MICmutant/MICWT) toward penicillin in strains expressing the Spy variants and the βla-Im7 L53A I54A folding biosensor containing a destabilized Im7 variant. C, all newly obtained Spy variants showed higher activity in preventing the aggregation of DTT-reduced α-LA (α-LA agg. Prev) compared with WT (n = 3, Student's t test). **p < 0.01; ***p < 0.001. Assay buffer contained 100 mm NaCl. D, variants with higher affinity (lower Kd) toward Im7 H40W L53A I54A as measured by bio-layer interferometry exhibit higher in vivo chaperone activity toward Im7 L53A I54A. E, thermodynamic stabilities of the Spy variants determined by urea-induced denaturation at 25 °C indicate that only three new variants (F29V, L32Q, and H96R) and the Q100L control are destabilized compared with WT. ΔGNU is the free energy difference between the unfolded and folded states. Error bars in (B), (C), and (D) (for MICnormal), S.D. of three independent measurements whose data points are shown; error bars in (D) (for Kd) and (E) are fitting errors.