Figure 5.
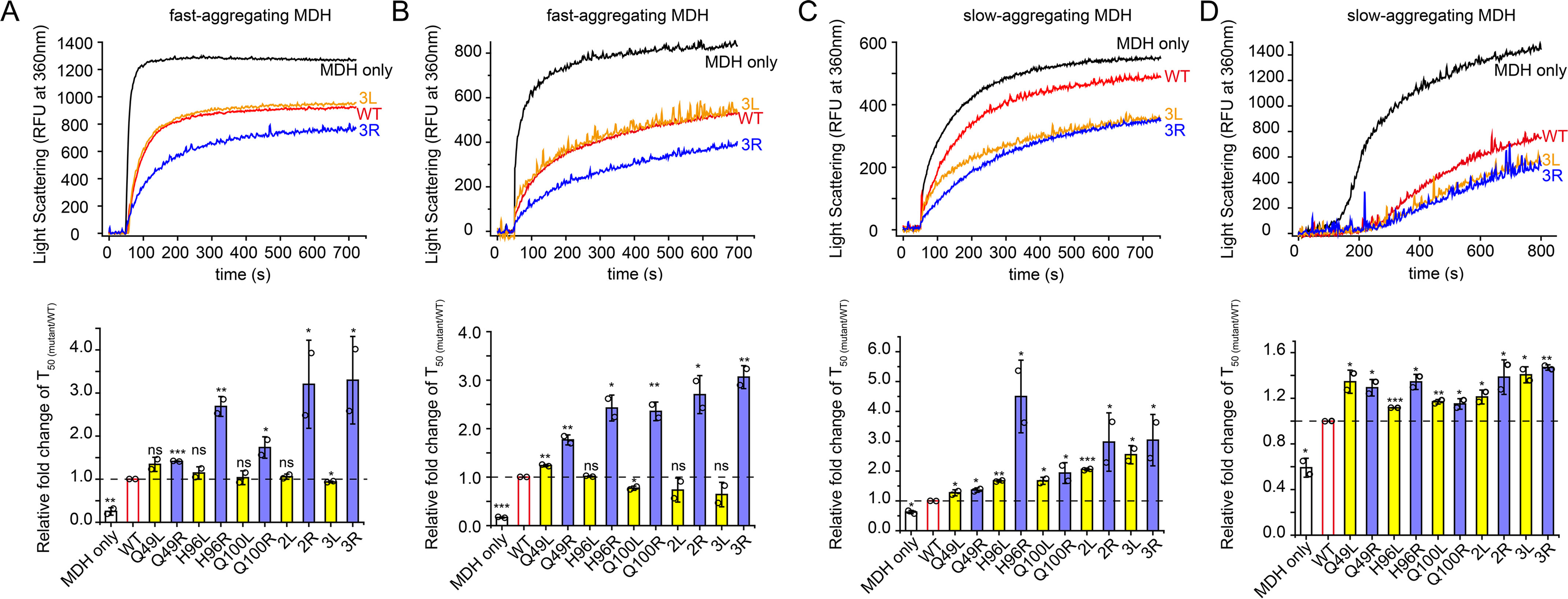
Anti-aggregation activities of leucine-substituted and arginine-substituted Spy variants toward MDH. A–C, MDH (80 µm) denatured in 8 m urea was diluted 160-fold into 40 mm HEPES-KOH buffer (pH 7.5) in the presence or absence of 0.05 µm Spy or different Spy variants at 32 °C (A), 25 °C (B), or 22 °C (C), and aggregation was monitored by light scattering at 360 nm. D, aggregation of 0.5 µm MDH denatured by heat at 43 °C in the absence or presence of 0.25 µm Spy WT or different Spy variants was monitored by light scattering at 360 nm. The upper panels in A–D, representative aggregation curves of MDH in the absence or presence of Spy WT or 3L/3R from duplicate measurements. The lower panel in A–D, relative anti-aggregation activities of Spy variants to Spy WT are expressed as the relative fold change of T50 (mutant/WT). T50 is the time to reach half of the maximum aggregation signal and is obtained by fitting the aggregation curves with the Hill equation with three parameters. For fast-aggregating MDH denatured by urea at 32 °C (A) or 25 °C (B), the arginine-substituted variants (blue) still show enhanced activities; however, the leucine-substituted variants (yellow) are only as active as the WT (red) (n = 2, Student's t test). For slow-aggregating MDH denatured by urea at 22 °C (C) or by heat at 43 °C (D), all arginine-substituted variants (blue) and leucine-substituted variants (yellow) performed better than Spy WT (n = 2, Student's t test). *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. Error bars, S.D. of two independent measurements.
