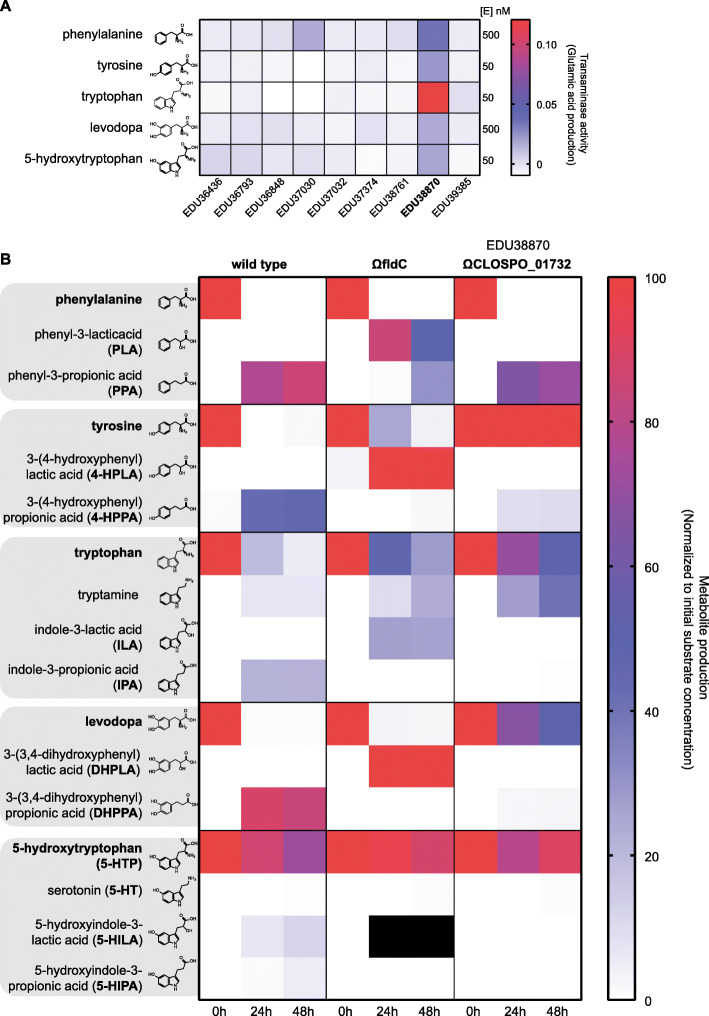
Fig. 2.
Identification of the aromatic amino transferase initiating the deamination pathway. In order to identify which aminotransferase is responsible for the initial transaminase reaction, all class I/II aminotransferases were cloned and purified to test the activity against (N)PAAAs. a Transaminase activity (production of glutamate) for all substrates is depicted. EDU38870 (CLOSPO_01732) was involved in all transaminase reactions. EDU37030 showed similar activity as EDU38870, for phenylalanine. Experiment was performed in technical duplicates to screen for candidate genes for mutagenesis in C. sporogenes. b Targeted metabolic quantification of deamination products from CSWT, CSΩfldC, and CSΩCLOSPO_01732 reveals that EDU38870 is involved in the transamination of all for all tested (N)PAAAs. All quantified deamination products are normalized to their initial substrate concentration, and the data represents 3 independent biological replicates. Corresponding values are reported, and metabolite concentration differences between WT and ΩfldC or ΩCLOSPO_01732 were tested for significance using Student’s t test, in Additional File 1: Table S1. Black squares indicate that quantification was not possible because of a coeluting HPLC-ED peak. As no commercial standards are available for 5-HILA and 5-HIPA, the peaks were quantified assuming a similar ED-detector response as for 5-HTP