Abstract
The aim of the present study was to investigate the probable effects of metformin plus vildagliptin on the oxidative stress index (OSI) in patients with type II diabetes mellitus (T2DM). In this case–control study, 44 patients with T2DM on either metformin monotherapy (n = 24) or metformin plus vildagliptin (n = 20) were compared with healthy controls (n = 20). Anthropometric and biochemical variables including body mass index, blood pressure profile, cardiac indices, lipid profile, fasting blood glucose, fasting serum insulin, and glycemic indices were assessed. Besides, total oxidant status (TOS), total antioxidant status (TAS), and OSI were determined. Patients with T2DM have higher risk of cardiometabolic changes compared with the control (P = 0.0001). TAS was lower while TOS and OSI were higher in patients with T2DM, as compared with the healthy controls (P < 0.001). TAS, TOS, and OSI were better in patients with T2DM on metformin plus vildagliptin therapy as compared with metformin monotherapy (P < 0.05). Therefore, this study concluded that metformin plus vildagliptin therapy is more effective than metformin monotherapy in attenuation of OSI in patients with T2DM.
Key words: Oxidative stress index, total antioxidant status, total oxidant status, type II diabetes mellitus
INTRODUCTION
Type II diabetes mellitus (T2DM) is a chronic endocrine disorder due to defect in the insulin action and/or secretion that is associated with cardiometabolic derangements. T2DM leads to chronic hyperglycemia, weight loss, and dyslipidemia.[1]
Prolonged hyperglycemia in T2DM induces oxidative stress (OS) via the generation of free radicals (FRs) through glucose auto-oxidation. In addition, hyperglycemia-induced protein glycosylation and cellular metabolism are considered as the additional sources of reactive oxygen species (ROS) and FRs.[2] Conversely, the levels of endogenous antioxidant capacity, chiefly glutathione and catalase, are reduced in patients with T2DM. Therefore, high ROS and FRs with low total body antioxidant status (TAS) in patients with T2DM may augment the hazard of OS injury and associated diabetic complications.[3] It has been reported that pancreatic β-cells have low antioxidant resistance capacity; therefore, it is highly vulnerable to the risk of OS injury.[4] Amid different literature survey, various studies show that OS is concerned and linked with the complications of T2DM. Prolonged untreated OS in patients with T2DM leads to endothelial dysfunction (ED), insulin resistance (IR), impaired pancreatic β-cells, and lipid peroxidation.[5] Moreover, OS and ED are interrelated in the progression of cardiometabolic complications in T2DM patients.[6] Hyperglycemia-induced microvascular complications are linked to the accumulation of sorbitol and fructose with the formation of advanced glycation end-products within endothelium, leading to ED.[2]
It has been shown that liver and adipose tissue are the major sources of ROS in diabetic patients since high hydrogen peroxide which is generated by adipose tissue is associated with the reduction of endogenous antioxidant capacity in induced T2DM in animal model study. What's more, triglyceride (TG) accumulation in the liver induces lipid peroxidation, and hepatic IR.[7]
Moreover, both of glucotoxicity and lipotoxicity impair pancreatic β-cells function via the generation of ROS, which suppress insulin mRNA and induction of apoptosis. Therefore, antioxidant therapy may improve glucose indices via decreasing the effect of ROS on the pancreatic β-cells.[8] Into the bargain, reduction of circulating free fatty acids improves insulin secretion and pancreatic β-cells function in obese T2DM patients.[9]
Metformin is a biguanide drug used as a first-line therapy of T2DM, which also has antioxidant effect.[10] As well, vildagliptin is an incretin-related drug that acts through the inhibition of dipeptidyl peptidase-4 and prolongs the half-life of glucagon-like peptide with subsequent suppression of glucagon secretion and stimulation of insulin.[11]
Therefore, the rationale of the present depends on the notion that glucolipotoxicity is the leading cause of OS in T2DM, and thus, diabetic pharmacotherapy due to their antioxidant effects may attenuate the associated OS.
Consequently, the aim of the present study was to discover the potential effects of metformin and/or vildagliptin on the OS and antioxidant capacity in patients with T2DM.
PATIENTS AND METHODS
Study design
In this case–control study, 44 patients with T2DM (18 females and 26 males) aged 42–64 years were recruited from the National Endocrinology Center during routine visits compared with 20 healthy controls (15 males and 5 females) matched with age and body weight. Full history and general physical examination with routine investigations were done by internist physician and endocrinologist. The recruited patients and healthy controls were subdivided into:
Healthy controls (n = 20)
T2DM patients on metformin therapy (n = 24)
T2DM patients on metformin plus vildagliptin therapy (n = 20).
In this study, patients with T2DM for more than 6 months were included in this study. However, patients with pregnancy, lactation, psychiatric and mental disorders, hepatic dysfunction, end-stage kidney disease, thyroid disorders, active infection, and sepsis were excluded from the study.
Anthropometric variables
Body mass index (BMI) was estimated by specific equation, Wt (kg)/Ht (m2); blood pressure was measured at supine position from the left arm by automated sphygmomanometer by which both diastolic blood pressure (DBP) and systolic blood pressure (SBP) were estimated. However, pulse pressure (PP) and mean arterial pressure (MAP) were calculated by specific equations according to Al-Naimi et al.'s method.[12]
Biochemical variables
After an over-night fasting, a blood sample of 10 ml was drained from all patients and healthy controls, centrifugated at 3000/rpm, and stored at −20°Ċ till the time of analysis. Fasting blood glucose (FBG), glycated hemoglobin (HbA1c), and fasting serum insulin (FSI) were measured by using biochemical and enzyme-linked immunosorbent assay (ELISA) kit method, respectively. IR and pancreatic β-cell function were estimated by homeostatic model assessment 2 (HOMA-2).[13] Lipid profile which includes total cholesterol (TC), TG, and high-density lipoprotein (HDL) was measured by ELISA kit methods (Abcam 65390, USA). Besides, low-density lipoprotein (LDL), very LDL (VLDL), non-HDL-cholesterol (TC − HDL-c), atherogenic index ([AI] = log [TG/HDL]), cardiac risk ratio ([CRR] = TC/HDL), and cardiovascular risk index ([CVRI] = TG/HDL) were calculated according to Al-Kuraishy et al.'s method.[14]
Indeed, both total oxidant status (TOS) and TAS were measured by ELISA kit (human total oxidant, MBS162650, Mybiosource, and human total antioxidant, MBS168358, Mybiosource), and both were expressed as μmol/L. Therefore, OS index (OSI) was calculated as 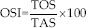 .
.
Data analysis
Data of the present study were presented as mean ± standard deviation. Unpaired Student's t-test was used to detect the level of significance between two unrelated groups; however, one-way ANOVA test with Bonferroni post hoc test was used to detect the level of significance among unrelated groups. Pearson's correlation test was used to detect the level of correlation. The level of significance was regarded when P < 0.05. All data analysis was done by Statistical Package for the Social Science (SPSS) (IBM SPSS 24.20,2018, Corp, Chicago, USA).
RESULTS
Study characteristics
In the present study, at first, eighty enrollments were recruited, 16 of them were excluded due to liver cirrhosis, brain tumors, and sepsis. The T2DM patients were subdivided into 24 patients on metformin monotherapy and 20 patients on metformin plus vildagliptin therapy compared with 20 healthy controls, as revealed in consort flow diagram of the present study [Figure 1].
Figure 1.
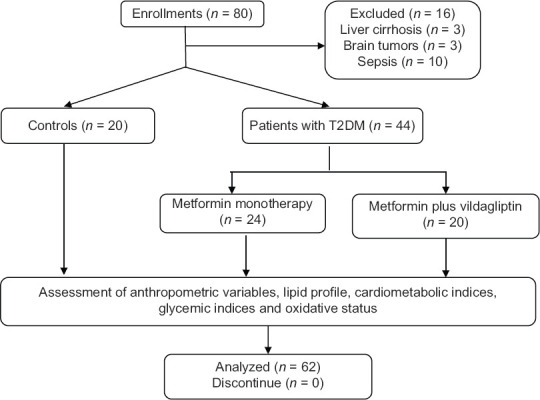
Consort flow diagram
The present study demonstrated that 65.62% of patients were males compared to 34.37% female. The duration of T2DM was 9.22 ± 2.41 years. The T2DM patients were associated with other concomitant diseases, such as dyslipidemia, hypertension, previous myocardial infarction, diabetic neuropathy, diabetic retinopathy, and diabetic nephropathy. Regarding the current pharmacotherapy of diabetic patients, 54.54% were on metformin therapy, while 45.45% were on metformin plus vildagliptin therapy and other therapies [Table 1].
Table 1.
Characteristics of the study
| Variables | n (%), mean±SD |
|---|---|
| n | 44 |
| Age (years) | 55.75±8.31 |
| Gender (male: female ratio) | 18 (40.90):26 (59.09) |
| Smoking | 26 (59.09) |
| Duration of T2DM (years) | 9.22±2.41 |
| Dyslipidemia | 40 (90.90) |
| Hypertension | 41 (93.18) |
| Previous MI | 8 (18.18) |
| Previous stroke | 3 (6.81) |
| Peripheral neuropathy | 11 (25.00) |
| Diabetic retinopathy | 8 (18.18) |
| Diabetic nephropathy | 5 (11.36) |
| Current therapy | |
| Metformin | 24 (54.54) |
| Metformin plus vildagliptin therapy | 20 (45.45) |
| Fenofibrate | 41 (93.18) |
| Statins | 10 (22.72) |
| Omega-3-fatty acids | 19 (43.18) |
| Anti-platelets | 11 (25.00) |
| ACEIs | 34 (77.27) |
| ARBs | 23 (52.27) |
| Anticoagulants | 4 (9.09) |
Data are expressed as n (%) mean±SD. MI: Myocardial infarction, ACEIs: Angiotensin-converting enzyme inhibitors, ARBs: Angiotensin receptor blockers, SD: Standard deviation, T2DM: Type II diabetes mellitus
Patients with T2DM of the present study have high risk of diverse cardiometabolic changes compared with control healthy subjects. BMI of diabetic patients was slightly higher than of control but not significant (P = 0.08). Blood pressure changes (SBP, DBP, PP, and MAP) were high in diabetic patients compared with the controls (P < 0.01). Glycemic indices (FBG, HbA1c, HOMA-IR, and HOMA-β cell function) were high in diabetic patients compared with the controls (P < 0.01). As well, LDL, VLDL, TC, and TG were higher) while HDL serum level was lower in diabetic patients compared with the controls (P < 0.01). Moreover, cardiac indices (CRR, AI, and CVRI) were high in diabetic patients compared with the controls (P < 0.01). Oxidative parameters were disorganized in patients with T2DM, TOS was higher in diabetic patients as compared with controls (P < 0.01), while TAS was low in diabetic patients as compared with the controls (P < 0.01). Furthermore, OSI was higher in diabetic patients compared with the controls (P < 0.01) [Table 2]. OSI of the patients with T2DM was not significantly correlated with patients' BMI (r = 0.22, P = 0.08). On the other hand, OSI was significantly correlated with blood pressure profiles and glycemic and cardiac indices. In this correlation, OSI was positively correlated with all of those variables, but it negatively correlated with HOMA-β, HDL, and TAS [Table 3]. OSI was higher in patients with T2DM on metformin monotherapy (3.21 ± 1.99) compared with healthy controls (1.02 ± 0.43). Further, OSI was higher in patients with T2DM on metformin plus vildagliptin (2.21 ± 1.12) compared with healthy controls. Patients with T2DM on metformin plus vildagliptin illustrated low OSI compared with T2DM on metformin monotherapy (P = 0.04) [Figure 2].
Table 2.
Cardiometabolic differences and oxidative stress in patients with type II diabetes mellitus regarding metformin monotherapy or metformin plus vildagliptin as compared with control
| Variables | Control (n=20) | T2DM + M (n=24) | T2DM + MV (n=20) |
|---|---|---|---|
| BMI (kg/m2) | 29.61±6.71 | 31.81±5.42 | 30.54±5.12 |
| SBP (mmHg) | 128.31±12.29 | 139.61±16.92× | 132.51±11.92* |
| DBP (mmHg) | 76.53±13.19 | 87.58±16.43× | 83.18±9.17* |
| PP (mmHg) | 51.78±8.65 | 52.03±8.31 | 52.85±8.55 |
| MAP (mmHg) | 93.79±7.49 | 104.92±9.63× | 98.31±9.45* |
| FBG (mg/dL) | 93.71±7.84 | 143.95±9.81× | 133.95±9.48* |
| HbA1c | 5.61±2.63 | 8.42±4.68× | 7.42±2.68# |
| FSI (μIU/mL) | 6.86±2.56 | 22.72±6.95× | 12.72±3.95* |
| HOMA-IR | 1.58±0.81 | 8.07±3.48× | 6.07±2.48* |
| HOMA-β% | 80.41±9.42 | 101.04±12.69× | 77.04±9.69 |
| TC (mg/dL) | 176.69±14.53 | 195.62±15.59× | 185.62±15.59* |
| TG (mg/dL) | 144.57±9.39 | 297.63±17.45× | 197.63±17.45* |
| HDL (mg/dL) | 55.91±5.61 | 39.63±8.29× | 49.63±8.29* |
| Non-HDL-c | 120.78±10.48 | 155.99±12.59× | 135.99±12.59 |
| LDL (mg/dL) | 91.90±8.73 | 96.50±9.51× | 92.50±9.51# |
| VLDL (mg/dL) | 28.91±5.94 | 59.62±6.41× | 33.62±6.41* |
| AI | 0.05±0.009 | 0.516±0.02× | 0.316±0.02* |
| CRR | 3.16±1.81 | 4.93±2.99× | 3.21±2.99# |
| CVRI | 2.58±1.72 | 7.51±2.63× | 5.51±2.63* |
| TOS (μmol/L) | 12.74±3.81 | 36.89±4.71× | 26.89±4.71 |
| TAS (μmol/L) | 1237.61±383.74 | 1145.89±293.51× | 1199.89±293.51# |
| OSI | 1.02±0.92 | 3.21±1.99× | 2.21±1.12# |
*P<0.01 as compared to T2DM+M, #P<0.05as compared to T2DM+M, ×P<0.01 as compared with control. Data are presented as mean±SD, unpaired t-test. BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PP: Pulse pressure, MAP: Mean arterial pressure, FBG: Fasting blood glucose, FSI: Fasting serum insulin, TC: Total cholesterol, TG: Triglyceride, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very low-density lipoprotein, AI: Atherogenic index, CRR: Cardiac Risk Ratio, CVRI: Cardiovascular risk index, TOS: Total oxidant status, TAS: Total antioxidant status, OSI: Oxidative stress index, M: Metformin, MV: Metformin plus vildagliptin, HbA1c: Glycated hemoglobin, SD: Standard deviation, T2DM: Type II diabetes mellitus
Table 3.
Pearson correlation of oxidative stress index with cardiometabolic changes in patients with type II diabetes mellitus
| Variables | R | P | 95% CI |
|---|---|---|---|
| BMI (kg/m2) | 0.22 | 0.08 | −0.027-0.442 |
| SBP (mmHg) | 0.39 | 0.01 | 0.159-0.58 |
| DBP (mmHg) | 0.41 | 0.007 | 0.183-0.596 |
| PP (mmHg) | 0.23 | 0.06 | −0.017-0.45 |
| MAP (mmHg) | 0.48 | 0.006 | 0.266-0.649 |
| FBG (mg/dL) | 0.84 | 0.0001 | 0.749-0.90 |
| HbA1c | 0.81 | 0.0001 | 0.704-0.88 |
| FSI (μIU/mL) | 0.78 | 0.001 | 0.704-0.88 |
| HOMA-IR | 0.93 | 0.0001 | 0.887-0.957 |
| HOMA-β% | −0.73 | 0.008 | −0.827-0.59 |
| TC (mg/dL) | 0.52 | 0.0001 | 0.314-0.679 |
| TG (mg/dL) | 0.93 | 0.0001 | 0.887-0.957 |
| HDL (mg/dL) | −0.84 | 0.0001 | −0.90-0.749 |
| Non-HDL-c | 0.48 | 0.007 | 0.266-0.649 |
| LDL (mg/dL) | 0.95 | 0.0001 | 0.919-0.969 |
| VLDL (mg/dL) | 0.85 | 0.0001 | 0.764-0.906 |
| AI | 0.88 | 0.0001 | 0.764-0.906 |
| CRR | 0.82 | 0.0001 | 0.719-0.887 |
| CVRI | 0.75 | 0.008 | 0.618-0.841 |
| TOS (μmol/L) | 0.97 | 0.0001 | 0.951-0.982 |
| TAS (μmol/L) | −0.99 | 0.0001 | −0.994-0.984 |
BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PP: Pulse pressure, MAP: Mean arterial pressure, FBG: Fasting blood glucose, FSI: Fasting serum insulin, TC: Total cholesterol, TG: Triglyceride, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very low-density lipoprotein, AI: Atherogenic index, CRR: Cardiac risk ratio, CVRI: Cardiovascular risk index, TOS: Total oxidant status, TAS: Total antioxidant status, OSI: Oxidative stress index, CI: Confidence interval
Figure 2.
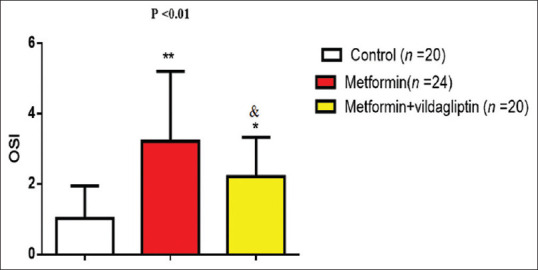
Oxidative stress index in patients with type II diabetes mellitus regarding the effects of diabetic pharmacotherapy. ∗P < 0.05 as compared to metformin monotherapy, ∗∗P < 0.01 as compared to the controls, P< 0.05 as compared to the controls
DISCUSSION
The current study revealed that T2DM patients had different cardiometabolic complications including hypertension, dyslipidemia, and OS as compared with healthy controls since OS causing hypertension and dyslipidemia may mediate T2DM-induced complications.[3]
Different reports show that hyperglycemia in T2DM is the main cause of OS through induction of FR and ROS generation that causes a variety of diabetic complications such as ED and systemic hypertension.[15] These findings are in contact with our results since high SBP, DBP, PP, and MAP were observed in patients with T2DM compared with the controls. In addition, our findings observed that systemic hypertension was correlated with the level of OS.
T2DM-induced OS leads to impairment of endothelial nitric oxide (NO) production with induction of inducible nitric oxide synthase (NOS) which aggravates the production of peroxynitrite as a replacement of NO. Peroxynitrite leads to the depletion of endothelial NOS and tetrahydrobiopterin causing ED and vascular damage through upregulation of pro-inflammatory cytokines.[16]
Likewise, high blood glucose in T2DM may provoke the expression of interleukin-6 and other pro-inflammatory adhesion molecules, so the inhibition of Poly (ADP-ribose) polymerases (PARPs) may prevent hyperglycemia-induced cardiovascular complications.[17]
Our findings demonstrated that glycemic indices were severely affected and correlated with OSI in patients with T2DM. Didangelos and Kantartzis established that hyperglycemia and hyperinsulinemia are concerned with the induction of OS, causing IR, impairment of pancreatic-β cell function, and insulin release. In the present study, high FSI and HOMA-β were higher to overcome of the developed IR.[18]
In addition, in the existing study, there was remarkable dyslipidemia which also correlated with OSI in patients with T2DM as revealed by Rasheed et al.'s study.[19] It has been reported that small-dense LDL, intermediate density lipoprotein (IDL), and chylomicron remnants are prone to oxidation with the induction of lipid peroxidation and induction of OS. Oxidized-LDL when binds to its receptors induces the release of chemokines and pro-inflammatory cytokines from activated macrophage. In turn, circulated and disseminated oxidized lipids play a role in the induction of OS.[20] Therefore, dyslipidemia collectively with OS contributes into cardiovascular complication in patients with T2DM which were seen noticeably in our study through high CRR, CVRI, and AI.
Furthermore, we observed significant OS in patients with T2DM compared with healthy controls. Reduction of TAS among diabetic patients could be attributed to hyperglycemia and lipid peroxidation. Similarly, the reduction of TAS reflects the activity of potential endogenous capacity to fight and stop underling oxidative injury. TAS provides a complete picture about the endogenous and exogenous antioxidant capacity; thus, it is more accurate than measuring of single antioxidant as they act synergistically against OS.[21]
In the present study, diabetic neuropathy, retinopathy, and nephropathy were observed with in patients with T2DM which were linked with the risk of OS since it leads to the diabetic microvascular and macrovascular complications through the induction of malondialdehyde (MDA), a byproduct of lipid peroxidation. Besides, MDA reacts reversibly or irreversibly with phospholipids and proteins, leading to microvascular complications.[22]
Concerning the effect of diabetic pharmacotherapy, in the present study, metformin or metformin plus vildagliptin might affect the oxidant and antioxidant status. Patients with T2DM who were treated with metformin plus vildagliptin illustrated low OSI compared with diabetic patients treated with metformin alone. Metformin therapy in T2DM have a potent antioxidant effect through direct scavenge of ROS or indirectly by inhibiting the production of superoxide anion, inhibition of intracellular NADPH oxidase, increasing the concentration of Vitamin E, improvement of endogenous antioxidant capacity, suppression of glucose autoxidation, and PARP pathway. Thus, metformin improves oxidative parameters as seen in our study. Similarly, metformin inhibits oxidative phosphorylation, thereby reducing cellular ATP levels, production of ROS, and induction of DNA repair.[23]
On the other hand, vildagliptin has significant antioxidant effect through reversing of ROS and endoplasmic reticulum stress.[24] Likewise, Ávila Dde et al.[24] point to the fact that vildagliptin improves ED in the experimental rats through promoting of autophagy and reduction of peroxynitrite serum levels.
What is more, 59.09% of our patients were active smokers which might be an additional factor in the induction of OS that led to high TOS and OSI. Al-Kuraishy et al.[25] confirmed that cigarette smoking leads to the induction of OS and the reduction of endogenous antioxidant capacity.
Besides, other drugs such as statins, fenofibrate, and ACEI that were used by our diabetic patients might affect body oxidant and antioxidant status. Effects of those drugs were excluded in the present since these drugs as documented from patients' history were used in an irregular pattern as most of those patients regarded these drugs are not essential in the management of T2DM.
It is inspiring to cognize that glucolipotoxicity is interrelated with OS in the initiation of T2DM-induced complications through a wide vicious cycle; thus, break-off components of this cycle by metformin and/or vildagliptin might be of great value in the prevention and attenuation of oxidative injury. Therefore, metformin and/or vildagliptin in addition to their potent antidiabetic effects improve TAC and reduce TOC and OSI in patients with T2DM.
CONCLUSION
Metformin and/or vildagliptin attenuate T2DM-induced OS through potentiating of TAC with the reduction of TOC and OSI. Therefore, combination of metformin and vildagliptin is an effectual combo against glucolipotoxicity and linked OS injury.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the registry database team and all medical staff members in the Department of Clinical Endocrinology for their cooperation.
REFERENCES
- 1.Al-Kuraishy HM, Al-Gareeb AI, Shams HA, Al-Mamorri F. Endothelial dysfunction and inflammatory biomarkers as a response factor of concurrent coenzyme Q10 add-on metformin in patients with type 2 diabetes mellitus. J Lab Physicians. 2019;11:317–22. doi: 10.4103/JLP.JLP_123_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dass AS, Narayana S, Venkatarathnamma PN. Effect of Vitamin E and omega 3 fatty acids in type 2 diabetes mellitus patients. J Adv Pharm Technol Res. 2018;9:32–6. doi: 10.4103/japtr.JAPTR_309_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lella M, Indira K. A comparative study of efficacy of atorvastatin alone and its combination with fenofibrate on lipid profile in type 2 diabetes mellitus patients with hyperlipidemia. J Adv Pharm Technol Res. 2013;4:166–70. doi: 10.4103/2231-4040.116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussien NR, Al-Naimi MS, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Sulfonylurea and neuroprotection: The bright side of the moon. J Adv Pharm Technol Res. 2018;9:120–3. doi: 10.4103/japtr.JAPTR_317_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdul-Hadi M, Naji M, Shams H, Sami O, Al-Kuraishy H, Al-Gareeb A. Erectile dysfunction and type 2 diabetes mellitus: A new twist. Int J Nutr Pharmacol Neurol Dis. 2020;10:43–9. [Google Scholar]
- 6.Seif El-Din SH, El-Lakkany NM, Salem MB, Hammam OA, Saleh S, Botros SS. Resveratrol mitigates hepatic injury in rats by regulating oxidative stress, nuclear factor-kappa B, and apoptosis. J Adv Pharm Technol Res. 2016;7:99–104. doi: 10.4103/2231-4040.184594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin on metabolic profile: Versatility of dose-dependent effect. J Adv Pharm Technol Res. 2019;10:33–8. doi: 10.4103/japtr.JAPTR_330_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurudeeban S, Satyavani K, Ramanathan T, Balasubramanian T. Antidiabetic effect of a black mangrove species Aegiceras corniculatum in alloxan-induced diabetic rats. J Adv Pharm Technol Res. 2012;3:52–6. doi: 10.4103/2231-4040.93560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Kuraishy HM, Al-Gareeb AI, Waheed HJ, Al-Maiahy TJ. Differential effect of metformin and/or glyburide on apelin serum levels in patients with type 2 diabetes mellitus: Concepts and clinical practice. J Adv Pharm Technol Res. 2018;9:80–6. doi: 10.4103/japtr.JAPTR_273_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Samerraie AY, Hamada MT, Al-Kuraishy HM. Effects of metformin on omentin levels in a newly diagnosed type II diabetes mellitus: Randomized, placebo controlled study. Mustansiriya Med J. 2016;15:49–56. [Google Scholar]
- 11.Brown K, Donato AA. In type 2 diabetes, early metformin plus vildagliptin reduced treatment failure vs. a stepwise approach. Ann Intern Med. 2020;172:JC23. doi: 10.7326/ACPJ202002180-023. [DOI] [PubMed] [Google Scholar]
- 12.Al-Naimi MS, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Berberine attenuates olanzapine induced-metabolic syndrome. J Pak Med Assoc. 2019;69(Suppl 3):S88–92. [PubMed] [Google Scholar]
- 13.Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases. 2018;6:31–9. doi: 10.3390/diseases6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin alone or in combination with omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J Basic Clin Pharm. 2016;8:8–14. doi: 10.4103/0976-0105.195080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadhim SS, Al-Windy SA, Al-Nami MS, Al Kuraishy HM, Al Gareeb AI. Statins improve periodontal disease–induced inflammatory changes and associated lipid peroxidation in patients with dyslipidemia: Two birds by one stone. J Int Oral Health. 2020;12:66. [Google Scholar]
- 16.Kadhim SS, Al-Windy SA, Al-Kuraishy HM, Al-Gareeb AI. Endothelin-1 is a surrogate biomarker link severe periodontitis and endothelial dysfunction in hypertensive patients: The potential nexus. J Int Oral Health. 2019;11:369. [Google Scholar]
- 17.Dludla PV, Dias SC, Obonye N, Johnson R, Louw J, Nkambule BB. A systematic review on the protective effect of N-acetyl cysteine against diabetes-associated cardiovascular complications. Am J Cardiovasc Drugs. 2018;18:283–98. doi: 10.1007/s40256-018-0275-2. [DOI] [PubMed] [Google Scholar]
- 18.Didangelos T, Kantartzis K. Diabetes and heart failure: Is it hyperglycemia or hyperinsulinemia? Curr Vasc Pharmacol. 2020;18:148–57. doi: 10.2174/1570161117666190408164326. [DOI] [PubMed] [Google Scholar]
- 19.Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI, Hussien NR, Al-Nami MS. Effects of diabetic pharmacotherapy on prolactin hormone in patients with type 2 diabetes mellitus: Bane or Boon. J Adv Pharm Technol Res. 2019;10:163. doi: 10.4103/japtr.JAPTR_65_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Kuraishy HM, Al-Gareeb AI. Potential effects of pomegranate on lipid peroxidation and pro-inflammatory changes in daunorubicin-induced cardiotoxicity in rats. Int J Prev Med. 2016;7:85. doi: 10.4103/2008-7802.184314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Kuraishy HM, Hussian NR, Al-Naimi MS, Al-Gareeb AI. Statins role in vitiligo: A mini-review. Turk J Dermatol. 2020;14:1. [Google Scholar]
- 22.Al-Kuraishy HM, Al-Gareeb AI. Eustress and malondialdehyde (MDA): Role of panax ginseng: Randomized placebo controlled study. Iran J Psychiatry. 2017;12:194–200. [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Kuraishy HM, Al-Gareeb AI. Erectile dysfunction and low sex drive in men with type 2 DM: The potential role of diabetic pharmacotherapy. J Clin Diagn Res. 2016;10:FC21–6. doi: 10.7860/JCDR/2016/19971.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ávila Dde L, Araújo GR, Silva M, Miranda PH, Diniz MF, Pedrosa ML, et al. Vildagliptin ameliorates oxidative stress and pancreatic beta cell destruction in type 1 diabetic rats. Arch Med Res. 2013;44:194–202. doi: 10.1016/j.arcmed.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. J Lab Physicians. 2018;10:276–82. doi: 10.4103/JLP.JLP_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


