Figure 1.
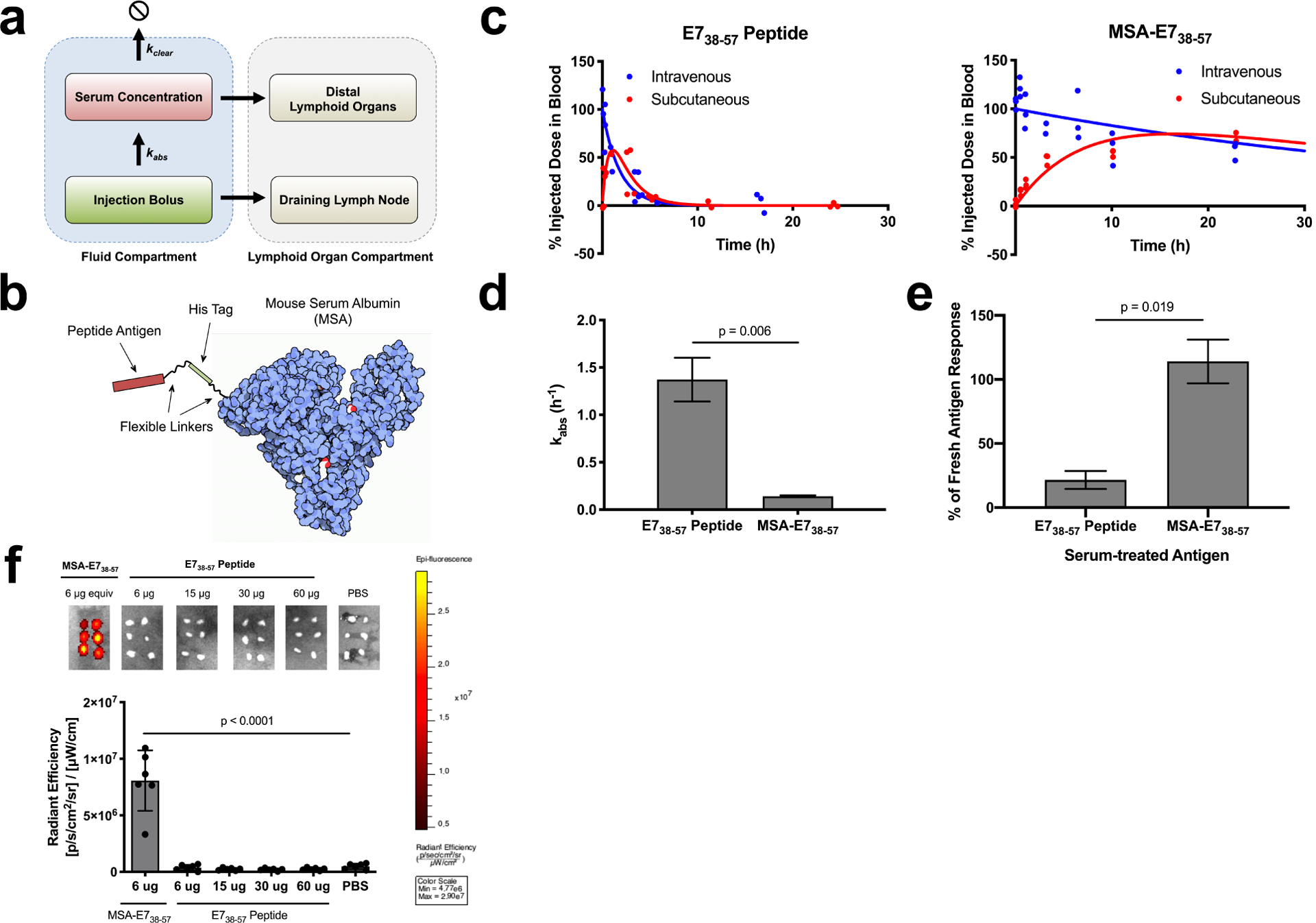
Albumin fusion enhances the bioavailability of antigen in the dLN. (a) Schematic of pharmacokinetic model describing absorbance rate (kabs) and clearance rate (kclear), which determine bioavailability in lymphoid organs. (b) Schematic of MSA-E738–57 protein design. (c) FITC labeled E738–57 or MSA-E738–57 was injected either subcutaneously or intravenously in mice (n = 3 mice per group). <10 μl blood draws were used to quantify antigen concentration in serum over the course of 24 hours following injection; data was used to determine pharmacokinetic model fits, shown with solid lines. (d) Calculated kabs rates for E738–57 peptide and MSA-E738–57 (fit data ± SE). (e) Splenocytes from E738–57-vaccinated mice were restimulated in the presence of brefeldin A with indicated antigen, either fresh or treated with 10% mouse serum for 24 hours. Shown is the percentage IFNγ response from serum-treated antigen restimulation compared to response from fresh antigen as measured by intracellular cytokine staining (n = 2 replicates). (f) FITC-labeled E738–57 or MSA-E738–57 was injected subcutaneously in mice at the indicated doses. 8 hours after injection, inguinal lymph nodes were excised and imaged on IVIS (n = 6 lymph nodes per group). Data are representative of two independent experiments. Statistical significance calculated using two-tailed t tests (d,e) or one-way ANOVA with Dunnett’s multiple comparisons test against PBS (f).
