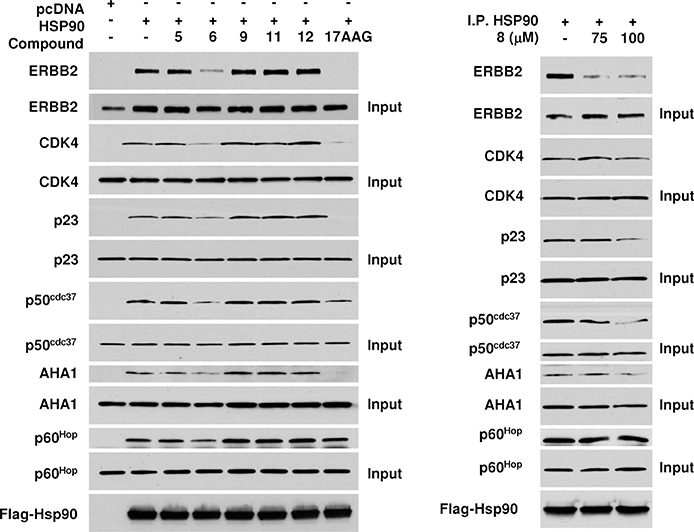
Figure 4.
Inhibition of Hsp90 chaperone function. Client and cochaperone binding to Hsp90 is inhibited by small molecules 6 and 8. COS7 cells were transfected with wild-type Flag–Hsp90. After incubation, cells were treated with 100 μM of the indicated Hsp90 inhibitor for 1 h. Then, cells were lysed, and proteins were immunoprecipitated (IP) by a Flag antibody-conjugated agarose. Indicated coprecipitating proteins were detected by immunoblotting.