Abstract
Background
Uncoded chronic kidney disease (CKD) is associated with poorer quality of care.
Aim
To ascertain the proportion and determinants of CKD, which have not been formally recorded (Read coded), and identify differences in management and quality-of-care measures for patients with coded and uncoded CKD.
Design and setting
Cross-sectional survey undertaken in an ethnically diverse adult population using primary care electronic health records (EHRs) from GP clinics in Lambeth, South London, UK.
Method
Multivariable logistic regression analysis examined the association of demographic factors, selected comorbidities, deprivation, and cardiovascular disease risk management in CKD, with coding status as outcome.
Results
In total, the survey involved 286 162 adults, of whom 9325 (3.3%) were identified with CKD stage 3–5 (assigned as CKD based on estimated glomerular filtration rate [eGFR] values). Of those identified with CKD, 4239 (45.5%) were Read coded, and 5086 (54.5%) were uncoded. Of those identified with CKD stage 3–5, individuals aged ≥50 years were more likely to be coded for CKD, compared with those aged <50 years. Lower levels of coding were independently associated with deprivation and black Caribbean, black African, South Asian, and non-stated ethnicities, compared with white ethnicity. Prescribed statin and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker medications were associated with increased odds of coded CKD.
Conclusion
This study found that >50% of CKD was uncoded and, for those patients, quality of care was lower compared with those with coded CKD. Future research and practices should focus on areas of greater deprivation and targeted initiatives for those aged <50 years and of black African, black Caribbean, South Asian, or non-stated ethnic groups. Possible areas for improvement include diagnostic coding support, automated CKD recording, and clinical decision support (based on adjusted eGFR results) in the GP clinical records.
Keywords: cardiovascular diseases, chronic kidney disease, clinical coding, ethnic groups
INTRODUCTION
Chronic kidney disease (CKD) is a major cause of morbidity and mortality in patients with hypertension, diabetes, and cardiovascular disease (CVD), and is a significant public health concern.1,2 Incidence and prevalence have substantially increased over the last decade, with a global prevalence of around 11%.3 More than 1.9 million people in England have diagnosed CKD; the total prevalence of adult CKD stage 3–5 was previously estimated to be 6%, and rises with age.4 Persistent albuminuria prevalence (with normal estimated glomerular filtration rate [eGFR]), which is considered to be CKD stage 1, has been estimated to be as high as 10%.5 Prevalence of diagnosed CKD stage 1 and 2 is estimated at 3.5–12% of adults aged ≥35 years.5 In addition, approximately 1 million people in the UK have, based on their eGFR results, CKD stage 3–5 but are not coded.6
In UK primary care, the Quality and Outcomes Framework (QOF) incentivises maintaining a CKD register that now includes classification of glomerular filtration rate (GFR) categories G3a to G5 (based on eGFR), but the blood pressure (BP) targets, angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) treatment, and albumin creatinine ratio (ACR) testing have been removed.7
CVD is the most common cause of mortality in later-stage CKD.8,9 Recognised risk factors for CKD include age, sex, ethnicity, hypertension, diabetes, CVD, smoking, and use of non-steroidal anti-inflammatory drugs (NSAIDs).10 CKD guidance includes BP management targets and statins for CVD prevention for all individuals with CKD stage 3–5.11,12 The systolic BP target in CKD is <140 mmHg (target range: 120–139 mmHg), and the diastolic BP target is <90 mmHg. For patients with type 2 diabetes (T2D) and/or a urinary ACR of >70 mg/mmol, BP targets are <130/80 mmHg.11 A recent meta-analysis found that GFR decline may be slowed with glycaemic and lipid-lowering control, but lacked a protective effect of antihypertensives; studies were, however, underpowered.13
Coding and management of disease
In the UK, GPs are responsible for the management of CKD and there are QOF financial incentives for coding and managing conditions in primary care; these are updated annually.7 Read codes and SNOMED Clinical Terms (CT) are used in the recording of clinical information in primary care,14 and include:
diagnostic codes;
measurements (such as laboratory test results, BP, height, and weight);
drug prescriptions; and
sociodemographic items.
How this fits in
| The coding of chronic kidney disease (CKD) is associated with improved quality of care and CVD risk management. This study identified health inequalities, with lower levels of coding in younger age groups (individuals aged <50 years); areas of greater deprivation; and black Caribbean, black African, South Asian, and non-stated ethnic groups compared with those of white ethnicity; such individuals would benefit from targeted improvement initiatives. |
The importance of CKD clinical coding — that is, formal diagnosis — in primary care was highlighted in the 2017 National Chronic Kidney Disease Audit, which recommended improved CKD coding.6 Patients who have uncoded CKD may have disease that is undiagnosed or missed.15 As 78% of those with CKD are managed in primary care, the audit noted that, without coding, a large number of people are at high risk of a lack of monitoring and appropriate follow-up, with increased risk of poor outcomes.6 However, presently ∼70% of patients with CKD are coded for CKD in primary care in England and Wales,16,17 and evidence suggests there is a positive relationship between coding and patient management.17 Other studies in different settings have shown lower levels of CKD coding to be associated with poorer health outcomes, including hospitalisation and lack of BP target achievement,18,19 and lower recording rates for hypertension and stroke.20 The authors decided to repeat the National Chronic Kidney Disease Audit (which comprised largely white, rural populations) in a multi-ethnic, urban setting,21 using more-recent data.
Objectives
The study aimed to:
determine proportion of uncoded CKD, based on eGFR values alone;
identify determinants of receiving a coded CKD diagnosis; and
identify differences in management and quality-of-care measures for patients with coded and uncoded CKD.
METHOD
Study setting and design
The study was undertaken in Lambeth, South London, and involved a cross-sectional survey of people with a Read code for CKD and/or reduced eGFR on the health record held by their GP. The authors determined coding status, risk factors, and measures of CKD management.
Data sources
This study utilised a dataset derived from general practice electronic health records (EHRs) for one inner-London borough, Lambeth DataNet (LDN), extracted in October 2013. LDN contains patient-level clinical data, prescribing data, laboratory data, and demographic information (including ethnicity, based on categories of the UK 2001 census), risk factors, and comorbidities. Demographic factors, comorbidities, and other quality-of-care measures were investigated in a multi-ethnic population identified as having CKD based on their eGFR. The eGFR was calculated from laboratory serum creatinine values using the modified four-variable Modification of Diet in Renal Disease (MDRD) equation, adjusted for sex and ethnic group.
Study population
The study was carried out using anonymised data from adult patients (aged ≥18 years) registered with 47 of 49 GP practices based in Lambeth, South London.
Identification of CKD coding status
Coded CKD status was determined using QOF CKD descriptive codes, plus codes for dialysis or renal transplantation (Supplementary Table S1), validated with biochemical evidence of CKD based on the latest two readings for eGFR levels <60 ml/min/1.73 m2 that were taken ≥3 months apart. Non-coded CKD was defined as individuals who fitted the criteria for biochemical CKD, but for whom there was no corresponding Read code entry.
Covariates
The authors examined factors such as age, sex, ethnicity, deprivation level (based on the Index of Multiple Deprivation 2015), and selected comorbidities that were likely to affect renal health outcomes, including hypertension, type 2 diabetes (T2DM), coronary heart disease (CHD), heart failure, and serious mental illness (SMI), using QOF registers at the time of the data extraction.22 SMI was selected as this is common in CKD,23 and studies report an association with increased CVD mortality and morbidity.24 Other measured factors affecting CKD progression and/or health outcomes were: systolic and diastolic BP control; statin, ACEi/ARB, and NSAID prescribed medication; and lifestyle factors, such as smoking and obesity. Ethnicity was self-reported and aggregated into seven categories: white, black African, black Caribbean, South Asian, Chinese, other, and non-stated. Non-stated was an available option for participants and does not include participants who did not answer. Systolic BP control was defined as <140 mmHg and diastolic BP control as <90 mmHg,11 based on the average of two latest readings. Proteinuria measurements (a measure of renal damage) were incomplete and, therefore, not included.
Outcomes
The authors examined the following metrics relevant to quality of care in CKD, comprising:
proportion of uncoded CKD (based on the eGFR);
determinants of receiving a coded CKD diagnosis; and
differences in management and quality-of-care measures between coded and uncoded CKD, based on CKD guidance from the National Institute for Health and Care Excellence, demographic factors, patient characteristics, and comorbidities.
Analysis
A cross-sectional assessment of people with biochemical CKD was used to assess factors associated with coding status in individuals with CKD, using Stata (version 15).
Logistic regression analysis was used to determine the association of demographic factors, selected comorbidities, and CVD risk-management measures with CKD coding status. Partly adjusted (adjusted for age group and sex) and fully adjusted (adjusted for age group, sex, and other covariates) analyses were conducted. The covariates adjusted for included ethnicity (white ethnicity as the reference group), locally based deprivation quintile, smoking status, comorbidities, and quality-of-care factors (CVD risk management and the prescribing of statin or ACEi/ARB medication).
RESULTS
Study population
The population comprised 286 162 adults from 47 out of 49 GP practices in Lambeth, South London. Details of patient characteristics can be seen in Table 1.
Table 1.
Uncoded and coded CKD Read codes among an adult population with biochemical CKD stage 3–5 (n = 9325), by selected clinical characteristics
| Variable | Total, N | Uncoded CKD | Coded CKD | P-value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | %a | n | %a | |||
| CKD stage ≥3 | 9325 | 5086 | 54.5 | 4239 | 45.5 | |
|
| ||||||
| Sex | ||||||
| Female | 5312 | 2858 | 53.8 | 2454 | 46.2 | |
| Male | 4013 | 2228 | 55.5 | 1785 | 44.5 | 0.10 |
|
| ||||||
| Age group, years | ||||||
| <50 | 1167 | 956 | 81.9 | 211 | 18.1 | <0.001 |
| 50–59 | 1464 | 1077 | 73.6 | 387 | 26.4 | |
| 60–69 | 1574 | 942 | 59.8 | 632 | 40.2 | |
| 70–79 | 2502 | 1130 | 45.2 | 1372 | 54.8 | |
| 80–89 | 2117 | 783 | 37.0 | 1334 | 63.0 | |
| ≥90 | 501 | 198 | 39.5 | 303 | 60.5 | |
|
| ||||||
| Ethnicity | ||||||
| White | 3847 | 1758 | 45.7 | 2089 | 54.3 | <0.001 |
| Black African | 1440 | 984 | 68.3 | 456 | 31.7 | |
| Black Caribbean | 2196 | 1297 | 59.1 | 899 | 40.9 | |
| South Asian | 540 | 260 | 48.1 | 280 | 51.9 | |
| Chinese | 62 | 30 | 48.4 | 32 | 51.6 | |
| Other ethnicity | 171 | 98 | 57.3 | 73 | 42.7 | |
| Non-stated | 206 | 129 | 62.6 | 77 | 37.4 | |
| Missing | 863 | 530 | 61.4 | 333 | 38.6 | |
|
| ||||||
| IMD quintile | ||||||
| 1 (least deprived) | 2252 | 1193 | 53.0 | 1059 | 47.0 | 0.42 |
| 2 | 2000 | 1102 | 55.1 | 898 | 44.9 | |
| 3 | 1600 | 886 | 55.4 | 714 | 44.6 | |
| 4 | 1741 | 968 | 55.6 | 773 | 44.4 | |
| 5 (most deprived) | 1726 | 932 | 54.0 | 794 | 46.0 | |
| Missing | 6 | 5 | 83.3 | 1 | 16.7 | |
|
| ||||||
| Comorbid conditions | ||||||
| Previous stroke | 678 | 293 | 43.2 | 385 | 56.8 | <0.001 |
| CHD | 1389 | 500 | 36.0 | 889 | 64.0 | <0.001 |
| Hypertension | 6318 | 2873 | 45.5 | 3445 | 54.5 | <0.001 |
| Type 2 diabetes | 2537 | 1079 | 42.5 | 1458 | 57.5 | <0.001 |
| Heart failure | 751 | 243 | 32.4 | 508 | 67.6 | <0.001 |
| Serious mental illness | 431 | 225 | 52.2 | 206 | 47.8 | 0.32 |
Percentages are calculated using the total figure for each variable as denominator, unless otherwise stated. CHD = coronary heart disease. CKD = chronic kidney disease. IMD = Index of Multiple Deprivation.
CKD coding
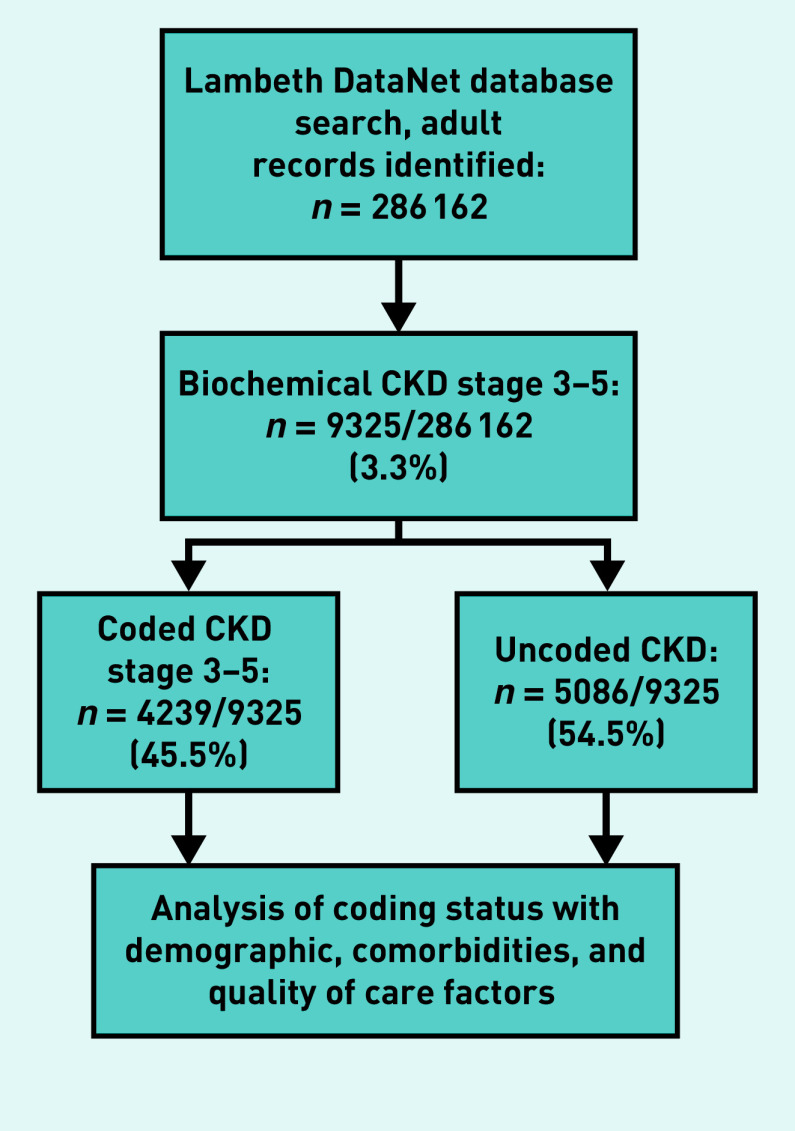
Of those identified with CKD (n = 9325), 4239 (45.5%) had a Read-coded CKD diagnosis, and 5086 (54.5%) were uncoded (validated with ethnicity-corrected eGFR). In total, 9325 out of 286 162 (3.3%) individuals were identified as having CKD based on eGFR values (Figure 1).
Figure 1.
Chronic kidney disease identification process. CKD = chronic kidney disease.
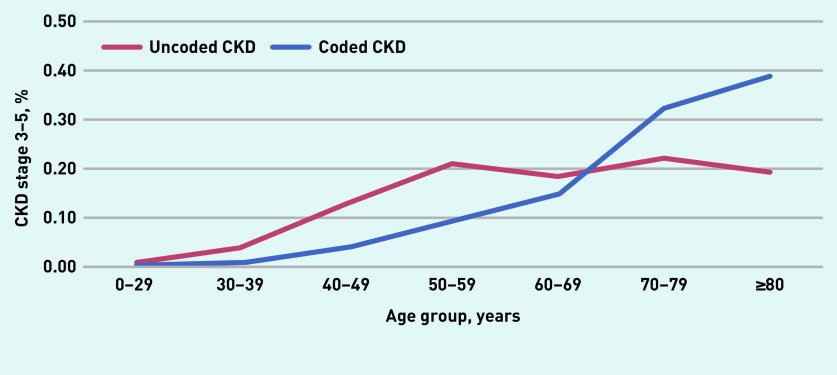
Table 1 summarises risk factors in 9325 patients with CKD, by coding status. The proportion of coded CKD was lower in younger age groups (aged <50 years). Figure 2 shows coding status by 10-year age group. CKD coding rose sharply in those aged ≥60 years. Coded CKD showed some inequalities between ethnic groups, for example, 54.3% of all CKD was coded in the white ethnicity group, but only 31.7% was coded in the black African and 40.9% in the black Caribbean groups, respectively.
Figure 2.
CKD Stage 3–5 coding status by age group for all adults (aged ≥18 years) in Lambeth DataNet. Denominator is total for uncoded and coded groups, N = 9325.
CKD = chronic kidney disease.
Table 2 shows quality-of-care measures, including BP control and pharmacotherapy. In patients with uncoded CKD, prescribed diuretic, ACEi/ARB, and statin medications were lower, but prescribed NSAID medications were higher than in patients with coded CKD.
Table 2.
Uncoded and coded CKD Read codes in an adult population with biochemical CKD stage 3–5, by selected quality-of-care characteristics
| Variable | Uncoded CKD (N = 5086) | Coded CKD (N = 4239) | P-value | ||
|---|---|---|---|---|---|
|
|
|
||||
| n | % | n | % | ||
| BP | |||||
| Mean systolic BP<140 mmHg | 1411 | 27.7 | 1720 | 40.6 | 0.80 |
| Missing | 2868 | 56.4 | 1550 | 36.6 | |
| Mean diastolic BP <90 mmHg | 2012 | 39.6 | 2535 | 59.8 | <0.001 |
| Missing | 2868 | 56.4 | 1550 | 36.6 | |
|
| |||||
| BMI | |||||
| >25 kg/m2 | 3262 | 64.1 | 2945 | 69.5 | 0.03 |
| Missing | 547 | 10.8 | 258 | 6.1 | |
|
| |||||
| Comorbidity history | |||||
| Current or ex-smoker | 702 | 13.8 | 458 | 10.8 | <0.001 |
| Missing | 21 | 0.4 | 3 | 0.1 | |
| Prescribed NSAID | 1394 | 27.4 | 884 | 20.9 | <0.001 |
| Prescribed diuretic | 1523 | 29.9 | 2066 | 48.7 | <0.001 |
| Prescribed ACEi/ARB | 2210 | 43.5 | 3183 | 75.1 | <0.001 |
| Prescribed statin | 2157 | 42.4 | 2849 | 67.2 | <0.001 |
ACEi = angiotensin-converting enzyme inhibitor. ARB = angiotensin receptor blocker. BMI = body mass index. BP = blood pressure. CKD = chronic kidney disease. NSAID = non-steroidal anti-inflammatory drug.
CKD burden and determinants of receiving a CKD diagnosis
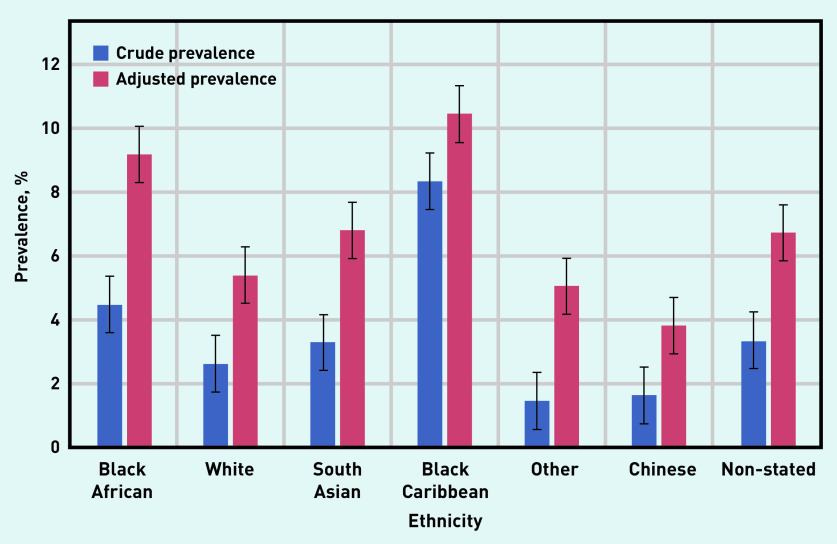
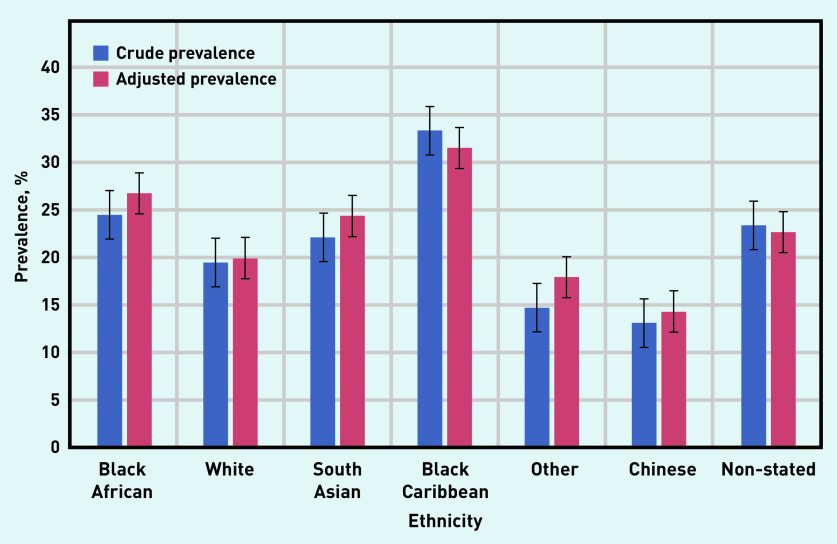
Of the 9325 individuals with CKD, the largest proportion was among those of black Caribbean and black African ethnicity, which remained after data were adjusted for age; age-standardised rates were produced using the mid-2013 England and Wales population estimate (Figures 3 and 4).25
Figure 3.
Prevalence of CKD stage 3–5 (coded and uncoded), by ethnic group in all adults (aged ≥18 years) in Lambeth DataNet. Age-adjusted data for the population, using the mid-2013 England and Wales adult population estimate.25
CKD = chronic kidney disease.
Figure 4.
Prevalence of CKD stage 3–5 (coded and uncoded), by ethnic group in adults aged ≥65 years in Lambeth DataNet. Age-adjusted data for the population, using the mid-2013 England and Wales adult population estimate.25
Table 3 shows the partially and fully adjusted analyses of those identified with CKD stage 3–5 adjusted for age group and sex. In the fully adjusted analyses, of those identified with CKD stage 3–5, being in an older age group was associated with increased odds of CKD being coded compared with those aged <50 years. Lower levels of coding were associated with all levels of deprivation compared with the quintile of least deprivation, and with black African, black Caribbean, South Asian, and non-stated ethnicity compared with white ethnicity. Some comorbidities, including hypertension, heart failure, and SMI, were associated with increased odds of CKD being recognised and coded (Table 3).
Table 3.
Partially and fully adjusted logistic regression analysis of the odds of being Read coded for CKD in an adult population with biochemical CKD stage 3–5
| Variable | Partially adjusteda OR (95% CI) | Fully adjustedb OR (95% CI2) | Fully adjustedbP-value |
|---|---|---|---|
| Age group, years | |||
| <50 | ref | ref | |
| 50–59 | 1.63 (1.35 to 1.97) | 1.26 (1.01 to 1.57) | 0.04 |
| 60–69 | 3.05 (2.55 to 3.65) | 1.86 (1.50 to 2.30) | <0.001 |
| 70–79 | 5.52 (4.66 to 6.53) | 2.89 (2.36 to 3.56) | <0.001 |
| 80–89 | 7.76 (6.53 to 9.24) | 3.88 (3.13 to 4.81) | <0.001 |
| ≥90 | 7.00 (5.54 to 8.84) | 4.06 (3.04 to 5.43) | <0.001 |
|
| |||
| Sex | |||
| Female | ref | ref | |
| Male | 1.05 (0.96 to 1.14) | 0.97 (0.88 to 1.07) | 0.54 |
|
| |||
| Ethnicity | |||
| White | ref | ref | |
| Black African | 0.66 (0.58 to 0.76) | 0.56 (0.48 to 0.66) | <0.001 |
| Black Caribbean | 0.71 (0.63 to 0.79) | 0.61 (0.54 to 0.70) | <0.001 |
| South Asian | 0.99 (0.82 to 1.20) | 0.81 (0.66 to 0.99) | 0.04 |
| Chinese | 0.97 (0.58 to 1.64) | 0.81 (0.46 to 1.42) | 0.46 |
| Other | 0.92 (0.66 to 1.29) | 0.83 (0.58 to 1.17) | 0.29 |
| Non-stated | 0.57 (0.42 to 0.77) | 0.58 (0.41 to 0.81) | 0.001 |
|
| |||
| IMD quintile | |||
| 1 (least deprived) | ref | ref | |
| 2 | 0.88 (0.77 to 1.00) | 0.83 (0.72 to 0.97) | 0.02 |
| 3 | 0.80 (0.70 to 0.92) | 0.76 (0.65 to 0.89) | 0.001 |
| 4 | 0.78 (0.69 to 0.89) | 0.79 (0.67 to 0.92) | 0.002 |
| 5 (most deprived) | 0.77 (0.68 to 0.89) | 0.76 (0.65 to 0.89) | 0.001 |
|
| |||
| Comorbidity history | |||
| Hypertension | 2.40 (2.17 to 2.65) | 1.43 (1.25 to 1.64) | <0.001 |
| Type 2 diabetes | 1.59 (1.44 to 1.75) | 1.08 (0.96 to 1.22) | 0.21 |
| Stroke | 1.22 (1.04 to 1.44) | 0.93 (0.77 to 1.12) | 0.46 |
| Serious mental illness | 1.05 (0.86 to 1.29) | 1.26 (1.00 to 1.64) | 0.05 |
| Heart failure | 2.00 (1.70 to 2.36) | 1.34 (1.11 to 1.62) | <0.001 |
| CHD | 1.67 (1.47 to 1.89) | 1.09 (0.94 to 1.26) | 0.27 |
|
| |||
| Quality-of-care factor | |||
| Statin prescribed | 2.03 (1.85 to 2.23) | 1.38 (1.23 to 1.55) | <0.001 |
| ACEi/ARB prescribed | 3.16 (2.88 to 3.47) | 2.24 (1.97 to 2.54) | <0.001 |
| NSAID prescribed | 0.80 (0.72 to 0.89) | 0.79 (0.71 to 0.89) | <0.001 |
| BMI >25 kg/m2 | 1.28 (1.15 to 1.41) | 1.07 (0.95 to 1.20) | 0.26 |
| Current smoker | 1.00 (0.88 to 1.15) | 1.03 (0.88 to 1.21) | 0.71 |
Adjusted for age and sex.
Adjusted for all covariates in the table. ACEi = angiotensin-converting enzyme inhibitor. ARB = angiotensin receptor blocker. BMI = body mass index. BP = blood pressure. CHD = coronary heart disease. CKD = chronic kidney disease. IMD = Index of Multiple Deprivation. NSAID = non-steroidal anti-inflammatory drug. OR = odds ratio.
Management and quality-of-care measures
Quality-of-care factors, including prescribed statins (adjusted odds ratio [OR] 1.38, 95% confidence interval [CI] = 1.23 to 1.55) and ACEi/ARB medications (adjusted OR 2.24, 95% CI = 1.97 to 2.54) were associated with increased odds of CKD coding. CKD coding was associated with lower use of prescribed NSAIDs (adjusted OR 0.79, 95% CI = 0.71 to 0.89).
In a separate analysis, target systolic BP control was also associated with an increased likelihood of being coded for CKD (adjusted OR 1.16, 95% CI = 1.04 to 1.30) (data not shown).
BMI was lower (P = 0.03) and proportion of current or ex-smokers higher (P = 0.001) in those not coded for CKD; however, BMI and smoking were not significantly associated with likelihood of CKD coding in the fully adjusted model (Table 3).
DISCUSSION
Summary
In this study of participants from ethnically diverse populations, the authors found that 54.5% of CKD was uncoded (that is, not formally recognised) and, for these patients, quality of care was lower. Younger adults (aged <50 years) with CKD were found to be less likely to be coded and, therefore, could be at risk of adverse CVD outcomes.
The age-adjusted burden of CKD showed that the largest proportion of cases were in black Caribbean and black African ethnic groups. It was found that participants of black Caribbean, black African, South Asian, and non-stated ethnicity were also less likely to be coded for CKD compared with those of white ethnicity, which may reflect physician recording bias. The association between uncoded CKD and black African and South Asian ethnicity is important, as it demonstrates an ethnic health inequality, which remained after adjusting for deprivation.
CKD coding was associated with comorbidities including hypertension, heart failure, and SMI. For those individuals coded for CKD, associated quality-of-care factors — such as prescribed statin and ACEi/ARB medications — were more likely. CKD coding was found to be associated with lower use of prescribed NSAIDs, possibly due to prescriber practice (less likely to prescribe NSAIDs because of concerns about renal toxicity).
Strengths and limitations
This study examined a number of coding determinants in a socioeconomically diverse adult population, and assessed CKD quality of care. A total of 47 of 49 general practices in Lambeth, South London, were included in this study, with a high representation of black and minority ethnic groups (42.9%; compared with the rest of England and Wales, 14.0%26), who are at increased risk of CKD and associated comorbidities.
The observed limitations are those often found with observational data and include misclassification, missing data, and unmeasured confounders, including GP practice factors. As >98% of patients in England are registered with a GP,27 data capture is high. However, the authors were unable to ascertain effect and direction of bias, because of missing data and the introduction of possible bias for BP control and BMI for patients with non-coded versus coded CKD.
Other limitations include selection (due to comorbidities and QOF coding) and survivor bias. In Lambeth, the population is younger and has greater levels of deprivation compared with the rest of the UK;28 as such, the authors would expect there to be higher levels of CKD in an older population.
This study reports an uneven prevalence of comorbidities (for example, stroke, CHD, and heart failure) between those who were coded and uncoded for CKD. This may be due to undercoding in the non-coded CKD population; however, because of QOF incentives, diseases such as stroke and CHD are likely to be coded, irrespective of CKD coding status.
It is likely that levels of hypertension are underdiagnosed, based on QOF disease registers,21 and BP recording was lower in non-coded CKD. Drug use was based on prescribed medications, although data on adherence were not available. Finally, long-term outcomes, such as mortality, were not examined as these were beyond the scope of this study.
Comparison with existing literature
The study presented here identified lower levels of CKD coding (45.5%) than those identified in the National Chronic Kidney Disease Audit (70%),17 but levels were similar to those of a recent study in Oxfordshire, OxRen, which found that 44.0% of (predominantly white) individuals living with CKD are undiagnosed without screening.29 This may reflect selection bias in participating GPs in the National Chronic Kidney Disease Audit, whereas the study presented here may be more reflective of busy urban practices. In contrast, one study has shown a higher prevalence of BP recording in black African and black Caribbean populations in the same Lambeth population.21
Older age (>50 years), male sex, diabetes, and hypertension are associated with CKD coding, and CKD coding is associated with receiving key primary care interventions recommended for CKD, including systolic BP control and pharmacotherapy.17 A study by Hull et al has shown hypotensive medication to be prescribed unequally among ethnic groups for any given range of BP control.30 In individuals with hypertension (both with and without CKD, adjusted by age and sex, and clustered by practice), the authors found that achieving the target BP (<140/90 mmHg) was better in patients of South Asian (OR 1.43, 95% CI = 1.28 to 1.60) ethnic groups and worse in black African (OR 0.79, 95% CI = 0.74 to 0.84) ethnic groups, compared with patients of white ethnicity.30 A systematic review of BP management in CKD populations, including minority ethnic groups, showed that quality improvement interventions can be effective at lowering BP and, potentially, at reducing CVD risk and slowing progression in CKD;31 in addition, modest improvements in systolic BP control (2.4 mmHg) were achieved through an audit education programme.32 People of minority ethnic groups are over-represented on renal replacement therapy (CKD stage 5);33 this may be because of some of the inequalities highlighted here.
The awareness of CKD coding in SMI is important, as this group is at increased risk of CVD mortality.24
Implications for practice
This study has highlighted the importance of CKD coding for improved disease management, as well as a health inequality. Possible areas for improvement include diagnostic coding support, automated CKD recording, and clinical decision support (based on adjusted eGFR results) in the GP clinical records.
The authors suggest that, as CKD coding is linked to QOF incentives around BP targets and pharmacotherapy, comorbidities and risk factor management are likely to improve in individuals who are coded for CKD.
Acknowledgments
This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Funding
Grace N Okoli was supported by a National Institute for Health Research lectureship (reference: CL-2017-17-007). No further funding was received for this study.
Ethical approval
Access to Lambeth DataNet (LDN) was granted by the LDN Steering Group and the Information Governance Committee at NHS Lambeth Clinical Commissioning Group (reference: LDNPIASMI19_01_18).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 2.Gansevoort RT. Too much nephrology? The CKD epidemic is real and concerning. A PRO view. Nephrol Dial Transplant. 2019;34(4):577–580. doi: 10.1093/ndt/gfy330. [DOI] [PubMed] [Google Scholar]
- 3.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Public Health England Chronic kidney disease prevalence model. 2014 https://www.renalreg.org/wp-content/uploads/2014/10/CKD-prevalence-final.pdf (accessed 6 Oct 2020). [Google Scholar]
- 5.Fat LN, Mindell J, Roderick P. Health Survey for England 2016: kidney and liver disease. 2017 NHS Digital. http://healthsurvey.hscic.gov.uk/media/63736/HSE2016-Adult-kid-liv.pdf (accessed 6 Oct 2020).
- 6.Nitsch D, Caplin B, Hull S, et al. National Chronic Kidney Disease Audit: national report (part 1) 2017 [Google Scholar]
- 7.NHS Digital Quality and Outcomes Framework (QOF) business rules v45.0 2020–2021 baseline release. 2020 https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/quality-and-outcomes-framework-qof/quality-and-outcome-framework-qof-business-rules/quality-and-outcomes-framework-qof-business-rules-v45.0-2020-2021-baseline-release (accessed 6 Oct 2020).
- 8.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 9.Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai WC, Wu HY, Peng YS, et al. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine (Baltimore) 2016;95(11):e3013. doi: 10.1097/MD.0000000000003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence . Chronic kidney disease in adults: assessment and management CG182. London: NICE; 2015. https://www.nice.org.uk/guidance/cg182/chapter/1-Recommendations#pharmacotherapy (accessed 16 Oct 2020). [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence . Cardiovascular disease: risk assessment and reduction, including lipid modification CG181. London: NICE; 2016. https://www.nice.org.uk/guidance/cg181/chapter/1-Recommendations#lipid-modification-therapy-for-the-primary-and-secondary-prevention-of-cvd-2 (accessed 6 Oct 2020). [Google Scholar]
- 13.Taylor KS, Mclellan J, Verbakel JY, et al. Effects of antihypertensives, lipid-modifying drugs, glycaemic control drugs and sodium bicarbonate on the progression of stages 3 and 4 chronic kidney disease in adults: a systematic review and meta-analysis. BMJ Open. 2019;9(9):e030596. doi: 10.1136/bmjopen-2019-030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer SA. Future of clinical coding. BMJ. 2016;353:i2875. doi: 10.1136/bmj.i2875. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Vargas PA, Tong A, Howell M, Craig JC. Educational interventions for patients with CKD: a systematic review. Am J Kidney Dis. 2016;68(3):353–370. doi: 10.1053/j.ajkd.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Kim LG, Caplin B, Cleary F, et al. Accounting for overdispersion when determining primary care outliers for the identification of chronic kidney disease: learning from the National Chronic Kidney Disease Audit. Nephrol Dial Transplant. 2017;32(Suppl_2):ii151–ii158. doi: 10.1093/ndt/gfw398. [DOI] [PubMed] [Google Scholar]
- 17.Kim LG, Cleary F, Wheeler DC, et al. How do primary care doctors in England and Wales code and manage people with chronic kidney disease? Results from the National Chronic Kidney Disease Audit. Nephrol Dial Transplant. 2018;33(8):1373–1379. doi: 10.1093/ndt/gfx280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwagami M, Caplin B, Smeeth L, et al. Chronic kidney disease and cause-specific hospitalisation: a matched cohort study using primary and secondary care patient data. Br J Gen Pract. 2018 doi: 10.3399/bjgp18X697973. [DOI] [PMC free article] [PubMed]
- 19.Hull SA, Rajabzadeh V, Thomas N, et al. Improving coding and primary care management for patients with chronic kidney disease: an observational controlled study in East London. Br J Gen Pract. 2019 doi: 10.3399/bjgp19X704105. [DOI] [PMC free article] [PubMed]
- 20.Walker N, Bankart J, Brunskill N, Bakker R. Which factors are associated with higher rates of chronic kidney disease recording in primary care? A cross-sectional survey of GP practices. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X561212. [DOI] [PMC free article] [PubMed]
- 21.Wu AS, Dodhia H, Whitney D, Ashworth M. Is the rule of halves still relevant today? A cross-sectional analysis of hypertension detection, treatment and control in an urban community. J Hypertens. 2019;37(12):2470–2480. doi: 10.1097/HJH.0000000000002192. [DOI] [PubMed] [Google Scholar]
- 22.NHS Digital Quality and Outcomes Framework (QOF) — 2013–14. 2014 Updated 2015. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/quality-and-outcomes-framework-qof-2013-14 (accessed 6 Oct 2020).
- 23.Iwagami M, Mansfield KE, Hayes JF, et al. Severe mental illness and chronic kidney disease: a cross-sectional study in the United Kingdom. Clin Epidemiol. 2018;10:421–429. doi: 10.2147/CLEP.S154841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Office for National Statistics Revised annual mid-year population estimates, UK: 2001 to 2010. 2013 https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/2013-12-17 (accessed 6 Oct 2020).
- 26.Office for National Statistics Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland. 2020 https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed 6 Oct 2020).
- 27.NHS Digital Patients registered at a GP practice August 2020. 2020 https://digital.nhs.uk/data-and-information/publications/statistical/patients-registered-at-a-gp-practice/august-2020 (accessed 6 Oct 2020).
- 28.NHS Lambeth Clinical Commissioning Group Public health report for Lambeth. Statistical bulletin 2013–14. 2014 https://www.lambeth.gov.uk/sites/default/files/ssh-annual_report_data_section.pdf (accessed 6 Oct 2020).
- 29.Hirst JA, Hill N, O’Callaghan CA, et al. Prevalence of chronic kidney disease in the community using data from OxRen: a UK population-based cohort study. Br J Gen Pract. 2020 doi: 10.3399/bjgp20X708245. [DOI] [PMC free article] [PubMed]
- 30.Hull S, Dreyer G, Badrick E, et al. The relationship of ethnicity to the prevalence and management of hypertension and associated chronic kidney disease. BMC Nephrol. 2011;12:41. doi: 10.1186/1471-2369-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher H, de Lusignan S, Harris K, Cates C. Quality-improvement strategies for the management of hypertension in chronic kidney disease in primary care: a systematic review. Br J Gen Pract. 2010 doi: 10.3399/bjgp10X502164. [DOI] [PMC free article] [PubMed]
- 32.de Lusignan S, Gallagher H, Jones S, et al. Audit-based education lowers systolic blood pressure in chronic kidney disease: the Quality Improvement in CKD (QICKD) trial results. Kidney Int. 2013;84(3):609–620. doi: 10.1038/ki.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udayaraj U, Pruthi R, Casula A, Roderick P. UK Renal Registry 16th annual report: chapter 6 demographics and outcomes of patients from different ethnic groups on renal replacement therapy in the UK. Nephron Clin Pract. 2013;125(1–4):111–125. doi: 10.1159/000360025. [DOI] [PubMed] [Google Scholar]