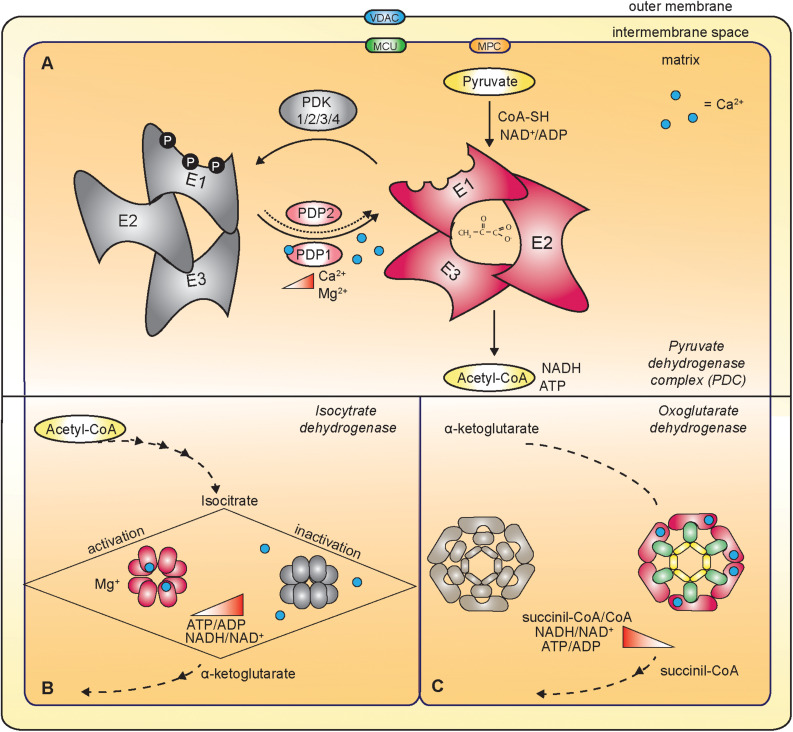
FIGURE 3.
Ca2+-dependent regulation of mitochondrial dehydrogenases. (A) The mitochondrial Ca2+ indirectly regulates the pyruvate dehydrogenase complex (PDC) that oxidizes pyruvate to acetyl-CoA. PDC is composed of multiple copies of three components: pyruvate decarboxylase (E1), dihydrolipoate acetyltransferase (E2) and dihydrolipoate dehydrogenase (E3). In response to increased mitochondrial [Ca2+], the Ca2+-dependent isoform of pyruvate dehydrogenase phosphatase 1 (PDP1) dephosphorylates E1, thus triggering PDC activation. (B) Ca2+ directly activates the isocitrate dehydrogenase (IDH). The ATP/ADP ratio negatively regulates the sensitivity of Ca2+ binding to IDH. (C) Oxoglutarate dehydrogenase (OGDH) is a multienzyme complex similar to the PDC. It is composed of dihydrolipoamide succinyl-transferase subunits (E2, green in the figure), oxoglutarate decarboxylase (E1, pink in the figure) and dihydrolipoamide dehydrogenase (E3, yellow in the figure). Ca2+-binding directly regulates the activity of the enzyme.