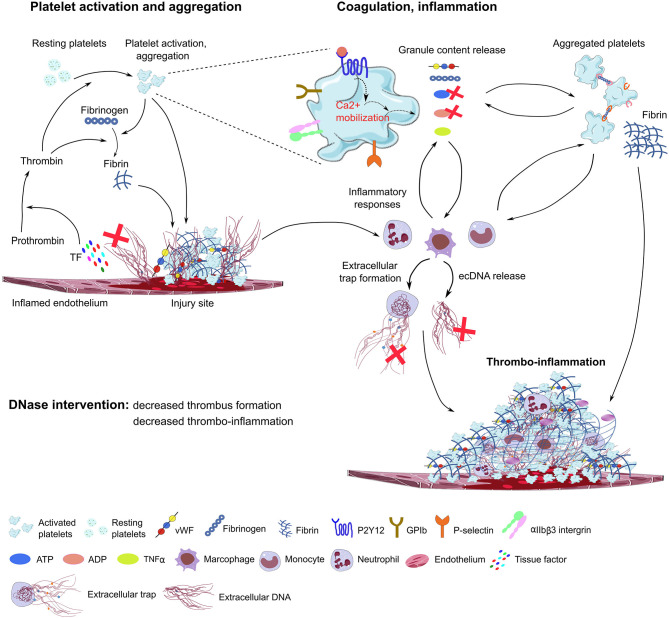
Figure 2.
Proposed model of DNase function in immunothrombosis. Damaged endothelial cells release tissue factor (TF) and ecDNA. TF activates the coagulation cascade, converting prothrombin to thrombin, which further activates platelets through PAR receptors. The ecDNA acts as DAMP and directly activates platelets and triggers inflammatory responses. The damaged endothelial layer exposes extracellular matrix proteins (collagen, laminin), and accumulates vWF, fibrinogen and other blood plasma proteins on the endothelial surface, further supporting platelet adhesion and activation through platelet specific glycoprotein receptors (GPIb, GPVI) and integrins (αIIbβ3, α2β1). During degranulation, second wave mediators (ATP, ADP, serotonin), extracellular matrix components (vWF, fibrinogen), and inflammatory cytokines are released by activated platelets, triggering thrombus formation and enhancing immune cell responses and NET formation. Platelet purinergic receptors (P2Y1, P2Y12) are activated by ADP, further promoting platelet aggregation and thrombus growth. P-selectin exposure on the plasma membrane of activated platelets increases procoagulant activity and supports platelet-immune cell interaction and NET formation. In the process of immunothrombosis, DNase could inhibit NETosis by fragmenting DNA within the NETs, thereby dissociating platelet-rich components from the endothelial surface, and inhibiting thrombus growth. DNAse may also inhibit purinergic signals in platelets and immune cells. TF, tissue factor; ec-DNA, extracellular deoxyribonucleic acid; vWF, von Willebrand Factor; ADP, adenosine diphosphate; ATP, adenosine triphosphate; TNFα, tumor necrosis factor alpha (TNFα); GPIb, glycoprotein Ib; GPVI, glycoprotein VI, DNase, deoxyribonuclease; ADP, adenosine diphosphate; ATP, adenosine triphosphate.