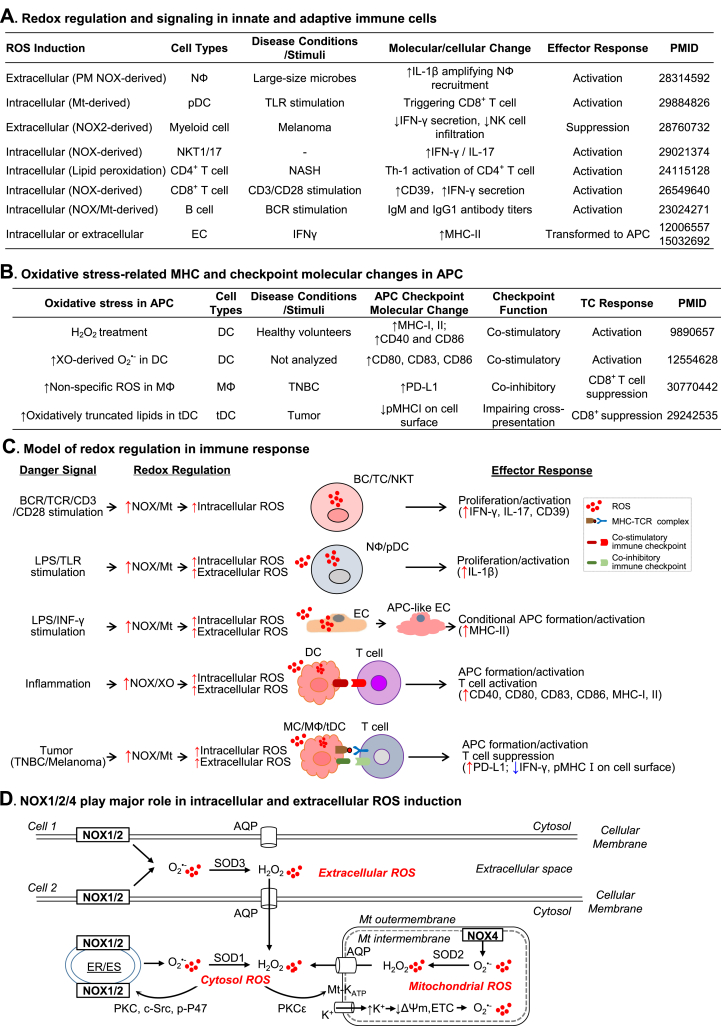
Fig. 4.
Redox regulation in innate-adaptive immunity interplay. Elevated Intracellular or extracellular ROS production is a critical metabolic change in immune responses. A. Redox regulation and signaling in innate and adaptive immune cells. In response to various stimuli or disease condition, immune cells display increased extracellular and intracellular ROS induction which are associated with inflammatory molecular-cellular changes and effector responses. B. Oxidative stress-related MHC and checkpoint molecular changes in APC. APC activation is associated with increased ROS or ROS-derived products which leads to immune checkpoint activation and T cell response. C. Model of redox regulation in immune response. Danger signals activate NOX which is responsible for increased intracellular and extracellular ROS production in immune or immune-like cells. The redox regulation mediates innate and adaptive immune cell activation, inflammatory response, APC formation/activation, and TC response via modulating the expression of major histocompatibility complex and immune checkpoint molecules. D. NOX1/2/4 play major role in intracellular and extracellular ROS induction. Plasma membrane-located NOX1/2 activation results in extracellular ROS production which can be dismutated by SOD3 to form H2O2 and then be diffused into the cytosol via water channel AQP. NOX1/2 located in PM/ER/ES can be activated via PKC-induced P47phox phosphorylation, resulting in intracellular O2•- generation which can be dismutated by SOD1 to form H2O2. NOX1/2-derived H2O2 can activate Mt-KATP via PKC-ε signaling, leading to the K+ influx, decrease of Mt membrane potential (ΔΨm), and ETC-derived ROS production. O2•- generated from Mt-located NOX4 is released into Mt matrix and dismutated by SOD2 to H2O2 which then be transported into cytosol via AQP.