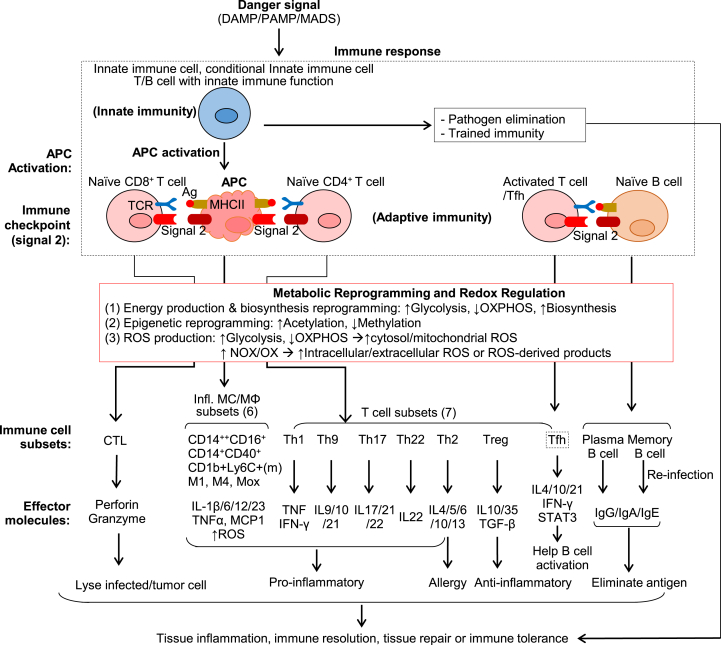
Fig. 5.
Innate-adaptive immunity interplay and redox regulation in immune response. Danger signals (DAMP/PAMP/MADS) induce immune response firstly via innate immune cell activation which leads to cytokine/chemokine production, trained immunity, pathogen elimination, and APC activation. The activated APC interacts with Naïve CD8+ and Naïve CD4+ T cell and function as a bridge to connect the innate and adaptive immune systems. Immune checkpoint molecule pairs (signal 2) determine the stimulatory or inhibitory immune response on T cell and APC. In the course of immune cell activation, active metabolic reprogramming leads to redox regulation and increased intracellular and extracellular ROS production. This results in immune subset differentiation. Naïve CD8+ T cell differentiates into CTL which lyse infected and tumor cells. Naïve CD4+ T cell differentiates into inflammatory subsets Th1/2/9/17/22, anti-inflammatory subsets Treg, and Tfh subset which helps Naïve B cell/B2 to differentiate into a plasma cell and the memory cell. MC/MΦ are classical APC and can be differentiated into anti-inflammatory subsets (not listed) and pro-inflammatory subsets (CD14++CD16+, CD14+CD40+, CD1b + Ly6C+(m), M1, M4, Mox). Immune cell subsets produce distinguish effector molecules which contribute to tissue inflammation or repair.