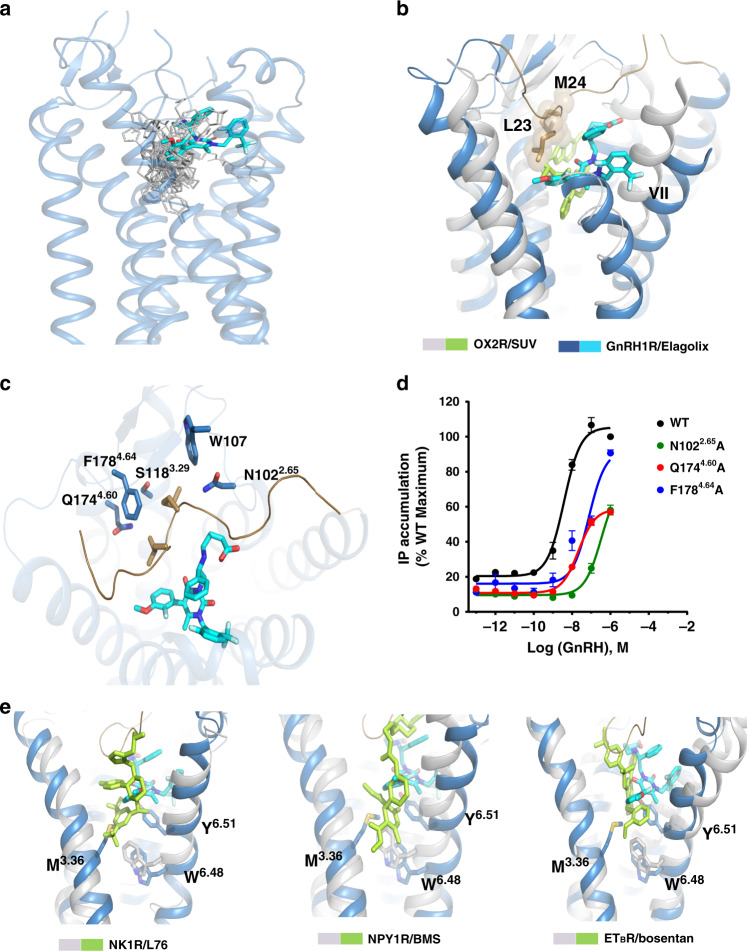
Fig. 3. Unusual orthosteric pocket of GnRH1R.
a Position comparison of elagolix in GnRH1R with small-molecule ligands in class A GPCRs family. The elagolix is shown as stick with cyan carbons, and the ligands from other GPCRs are shown as gray lines. b Structural superposition of GnRH1R with OX2R (gray cartoon, PDB ID: 4S0V), the ligand SUV (suvorexant) of OX2R is shown as green stick. In GnRH1R, two residues L23 and M24 (shown as cyan sticks with sphere) of the N terminus occupy the enlarged orthosteric pocket. c Key residues (sky blue sticks) within 4 Å of both L23 and M24 in GnRH1R. d Dose-dependent responses of GnRH1R WT and mutants (N1022.65A, Q1744.60A, and F1784.64A) to GnRH agonist determined by IP assays. See Supplementary Table 2 for detailed statistical evaluation. HEK293T were transiently transfected with the wild-type or mutant receptors (without PGS) and IP accumulation was measured after stimulation with GnRH ligands for 2 h. EC50 values are expressed as means ± SEM (n = 3) at three times independent experiment repeats with similar results. e Structural superposition of the elagolix-bound GnRH1R with L76 (L76075)-bound NK1R (PDB ID: 6E59), BMS (BMS-193885)-bound NPY1R (PDB ID: 5ZBH), and Bosentan-bound ETBR (PDB ID: 5XPR), respectively. Those three ligands (L76, BMS, and Bosentan) shown as green stick bound deeply in their corresponding receptor (shown as gray cartoon) orthosteric site. The toggle switch residue W6.48 is shown as a stick in all four receptors and both M3.36 and Y6.51 are shown in sky blue sticks forming the bottom wall of orthosteric pocket of GnRH1R.