FIGURE 1.
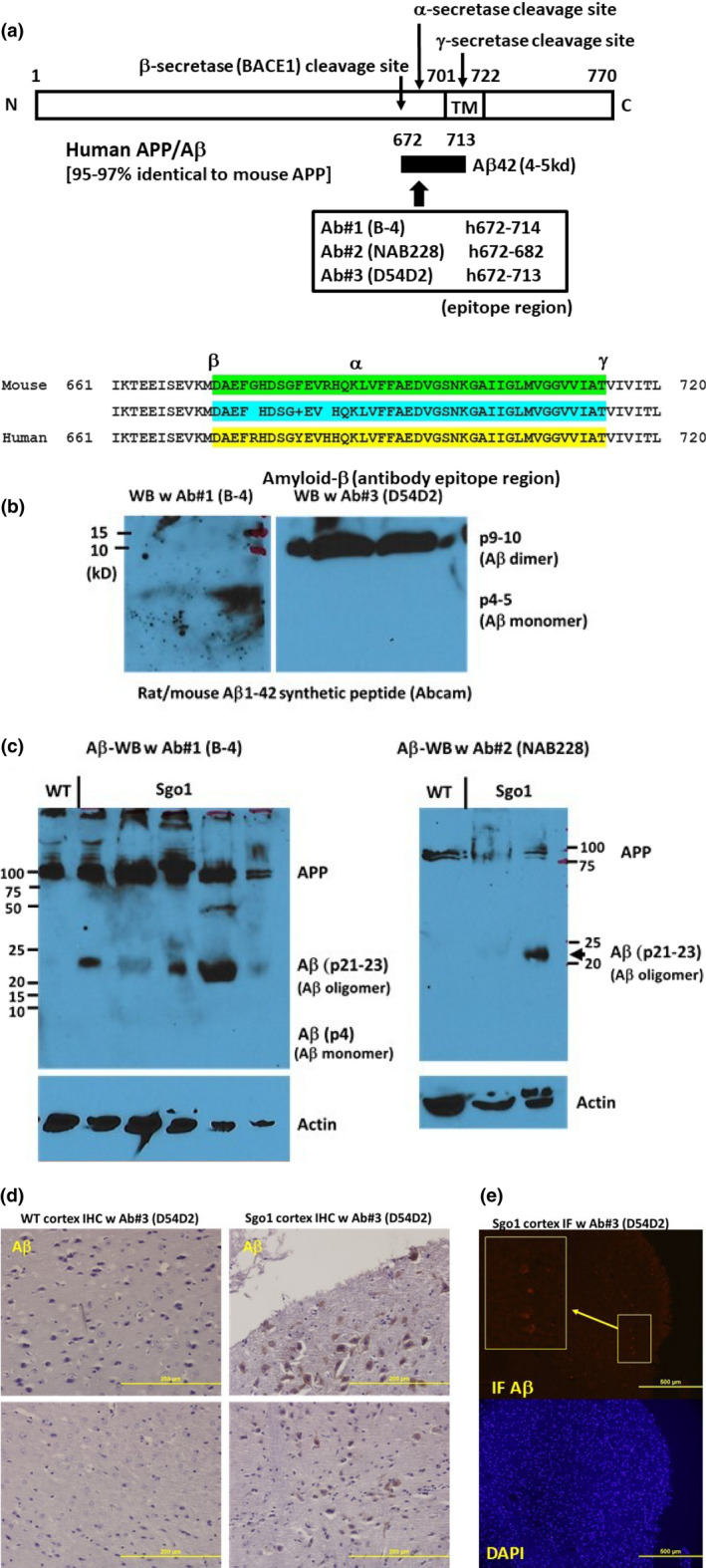
Cerebral amyloid‐β is accumulated in aged (24 month‐old) Sgo1−/+ mice. (a) Human/mouse APP structure. Anti‐amyloid‐β antibodies used for this study were generated against human amyloid‐β, which is 97% identical to mouse amyloid‐β. In humans, B‐4 and NAB228 recognize both APP and amyloid‐β, while D54D2 preferentially recognizes amyloid‐β. (b) Synthetic rat/mouse amyloid‐β1−42 peptide was recognized by anti‐ amyloid‐β antibodies. Amyloid‐β can form SDS‐resistant oligomers that may expose epitope regions differently. B‐4 antibody preferentially recognized monomer (p4‐5), while D54D2 recognized dimer (p9‐10). (c) Twenty‐four‐month‐old Sgo1−/+ brain extracts showed amyloid‐β p21‐23. Both anti‐amyloid‐β antibodies, B‐4 (left panel) and NAB228 (right panel), detected APP and amyloid‐β p21‐23 in immunoblots. Immunoblots of extracts from age‐matched wild‐type mice detected only APP. (d) Twenty‐four ‐month‐old Sgo1−/+ brain showed amyloid‐β accumulation in IHC. Control age‐matched wild‐type mice did not show IHC‐positive staining. (e) Twenty‐four‐month‐old Sgo1−/+ brain showed amyloid‐β accumulation in IF. IF showed positive signals in Sgo1−/+ brain, consistent with IHC results. Enlarged panel shows the signals from cell bodies. Control wild‐type mice did not show clear signals with equalized image acquisition settings (not shown)
