FIGURE 5.
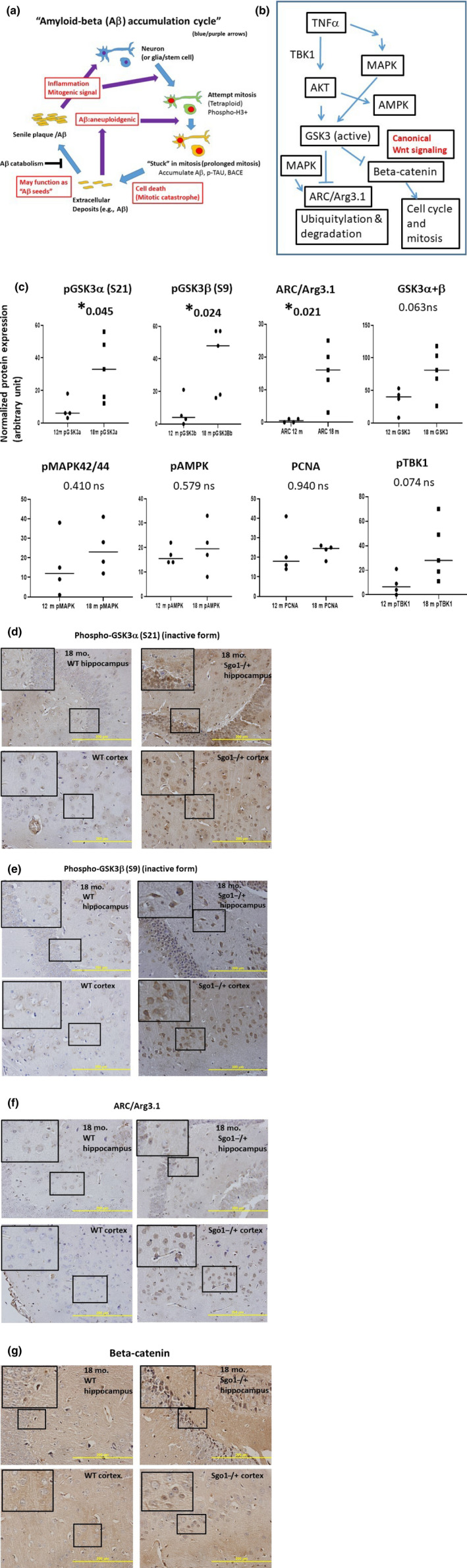
Inhibition of GSK3 α and β, accumulation of ARC/Arg3.1, and activation of Wnt signaling in amyloid‐accumulating Sgo1−/+. (a) The “amyloid‐β accumulation cycle” hypothesis (Rao et al., 2020). The “amyloid‐β accumulation cycle” hypothesis purports the occurrence of vicious cycles of events leading to amyloid‐β accumulation (see Introduction). Among a few mysteries in the hypothesis is the growth signaling driving the amyloid‐β accumulation cycle. (b) Key growth signaling pathways that are misregulated in human AD patients. AKT, AMPK, MAPK, and GSK3 are among the growth signaling misregulated in human AD and proposed to be involved in the disease process. GSK3 targets ARC/Arg3.1 and β‐catenin with ubiquitylation‐mediated proteolysis. (c) Phosphorylated GSK3 α and β (inactive forms) increased in Aβ‐accumulating Sgo1−/+. We tested components of the growth signaling in (b). Amounts of pGSK3α (S21) and pGSK3β (S9), inactive forms of GSK3, increased significantly in Aβ‐accumulating Sgo1−/+, while the total amount of GSK3 showed only a minor change. Consistently, ARC/Arg3.1 amount also significantly increased. pMAPK42/44, pAMPK, PCNA, and pTBK1 did not show significant change. (d) Nuclear accumulation of pGSK3α (S21) in Sgo1−/+. Consistent with immunoblots in (c) Aβ‐accumulating Sgo1−/+ showed accumulation of pGSK3α in the nucleus, both in the hippocampus and in the cortex. Age‐matched wild‐type showed no such pGSK3α accumulation. Enlarged panels for localization details. (e) Cytoplasmic accumulation of pGSK3β (S9) in Sgo1−/+. Aβ ‐accumulating Sgo1−/+ showed accumulation of pGSK3β in the cytoplasm, both in the hippocampus and in the cortex. Enlarged panels for localization details. (f) ARC/Arg3.1 was accumulated in the nucleo‐cytoplasm. ARC/Arg3.1 was accumulated in the nucleo‐cytoplasm, in both the hippocampus and the cortex, in Sgo1−/+. Enlarged panels for localization details. In wild‐type, IHC signals for ARC/Arg3.1 were much weaker, if any. (g) Another GSK3 target β‐catenin was enriched in the nuclei of Sgo1−/+, indicating Wnt signaling activation. Consistent with GSK3 inactivation, nuclear translocation of β‐catenin, a sign of canonical Wnt signaling activation and cell fate toward cell cycle and mitosis, was observed in Sgo1−/+ as distinct nucleo‐cytoplasmic signals in both the hippocampus and the cortex. β‐catenin IHC signals in wild‐type were weak, if any (enlarged panels)
