Fig 1.
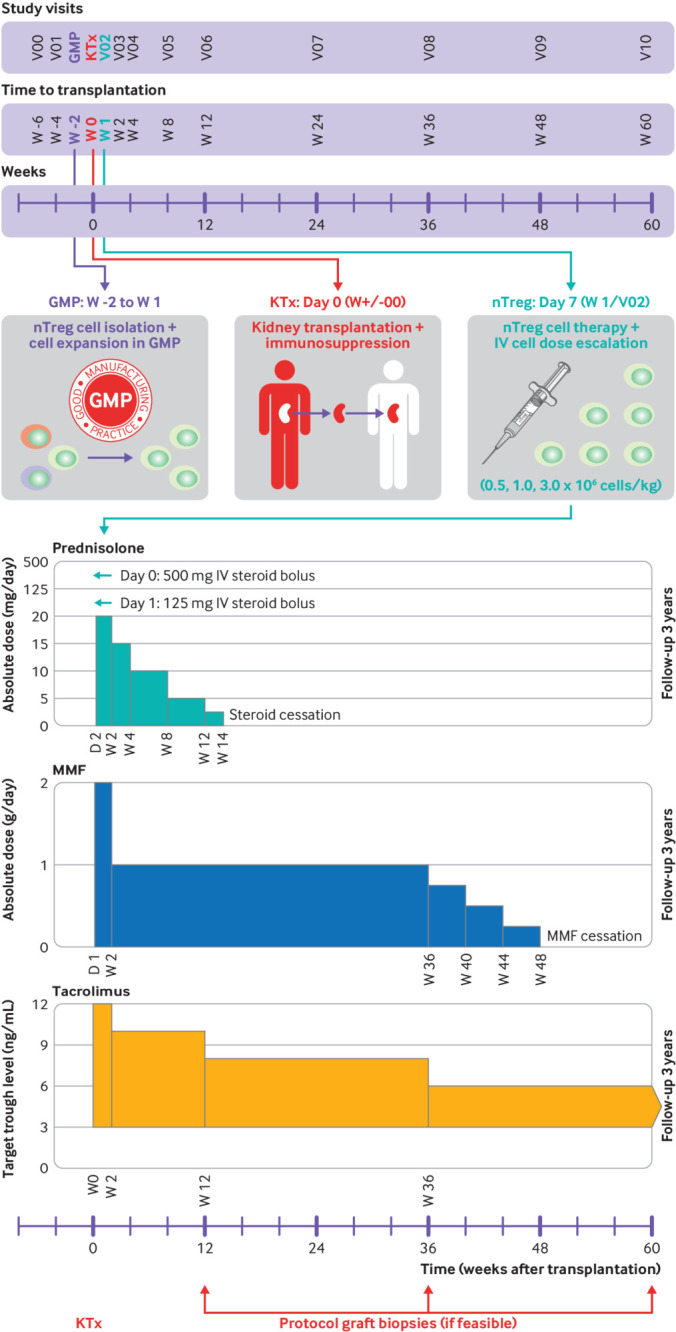
ONE study ONEnTreg13 clinical trial design. Upper panel: time schedule of ONEnTreg13 clinical trial: patient enrolment, cell collection, living donor kidney transplantation, nTreg adoptive cellular therapy (dose escalation of 0.5, 1.0, or 2.5-3.0×106 fresh cells/kg of body weight), and primary 60 week study follow-up. Lower panel: overview of protocol immunosuppressive regimen of ONEnTreg13 clinical trial with doses adjusted to specified levels at indicated time points: first steroid reduction until week 14, followed by MMF reduction at week 36-48, with continuation of tacrolimus monotherapy in nTreg group until study end at week 60 and three year follow-up. GMP=good manufacturing practice; IV=intravenous; KTx=kidney transplantation; MMF=mycophenolate mofetil; nTreg=natural regulatory T cell
