Abstract
OBJECTIVE
Weight loss diets may reduce serum urate (SU) by lowering insulin resistance while providing cardiometabolic benefits, something urate-lowering drugs have not shown in trials. We aimed to examine the effects of weight loss diets on SU and cardiometabolic risk factors.
RESEARCH DESIGN AND METHODS
This secondary study of the Dietary Intervention Randomized Controlled Trial (DIRECT) used stored samples from 235 participants with moderate obesity randomly assigned to low-fat, restricted-calorie (n = 85); Mediterranean, restricted-calorie (n = 76); or low-carbohydrate, non–restricted-calorie (n = 74) diets. We examined SU changes at 6 and 24 months overall and among those with hyperuricemia (SU ≥416 μmol/L), a relevant subgroup at risk for gout.
RESULTS
Among all participants, average SU decreases were 48 μmol/L at 6 months and 18 μmol/L at 24 months, with no differences between diets (P > 0.05). Body weight, HDL cholesterol (HDL-C), total cholesterol:HDL-C ratio, triglycerides, and insulin concentrations also improved in all three groups (P < 0.05 at 6 months). Adjusting for covariates, changes in weight and fasting plasma insulin concentrations remained associated with SU changes (P < 0.05). SU reductions among those with hyperuricemia were 113, 119, and 143 μmol/L at 6 months for low-fat, Mediterranean, and low-carbohydrate diets (all P for within-group comparison < 0.001; P > 0.05 for between-group comparisons) and 65, 77, and 83 μmol/L, respectively, at 24 months (all P for within-group comparison < 0.01; P > 0.05 for between-group comparisons).
CONCLUSIONS
Nonpurine-focused weight loss diets may simultaneously improve SU and cardiovascular risk factors likely mediated by reducing adiposity and insulin resistance. These dietary options could provide personalized pathways to suit patient comorbidity and preferences for adherence.
Introduction
Chronic hyperuricemia is a necessary precursor to develop gout, the most common form of inflammatory arthritis. Considered as a part of the insulin resistance syndrome (1,2), hyperuricemia and gout are both associated with a high burden of cardiometabolic morbidities as well as an increased risk of premature mortality (3,4). Although epidemiologic studies have linked various diet and lifestyle factors with serum urate (SU) concentrations and gout (5–7), evidence from dietary interventional trials are scarce. Traditionally, individuals with hyperuricemia or gout have been encouraged to follow a low-purine (i.e., low-protein) diet to reduce purine loading (2). However, the dietary purine source does not usually contribute >59 μmol/L (1 mg/dL) to SU concentrations (8). Furthermore, low-purine diets can lead to substitution of protein for refined carbohydrates (including fructose) and unhealthy fats (including trans and saturated fat), which could worsen gout’s cardiometabolic comorbidities by increasing adiposity, insulin resistance, and concentrations of plasma glucose, triglycerides, and LDL cholesterol (LDL-C) particles (2). As such, the low-purine approach is likely net harmful for long-term implementation, and it would be ethically challenging to justify a clinical trial of such duration. Moreover, large-scale pharmaceutical trials of drugs that substantially lowered SU concentrations to date have found no apparent cardiometabolic risk benefits, such as weight change, lipid profile, blood pressure, or renal function (9–12). A trial of 6,190 patients with gout found a 34% increased risk of cardiovascular deaths in the febuxostat group compared with allopurinol, leading to a Food and Drug Administration black box warning (9).
Conversely, there are healthful diets recommended by the American Heart Association/American College of Cardiology (13) and Dietary Guidelines for Americans 2015–2020 (14) that have been shown to improve cardiometabolic risks and risk factors. These diets may also reduce SU concentrations by lowering adiposity and insulin resistance (thereby enhancing uric acid excretion) (2,15,16); however, limited data on their impact on SU concentrations are available to date, particularly those arising from randomized trials. We therefore examined the effects of three healthful weight loss diets on SU concentrations and cardiometabolic risk factors in a secondary analysis of the Dietary Intervention Randomized Controlled Trial (DIRECT), hypothesizing that these diets would all improve SU concentrations and cardiometabolic risk factors (17).
Research Design and Methods
Direct
DIRECT was a randomized weight loss trial conducted in a workplace setting in Dimona, Israel, from July 2005 to July 2007 (17). Briefly, DIRECT compared the effects of three diets (low-fat, restricted calorie; Mediterranean, restricted calorie; and low-carbohydrate, non-restricted calorie) on weight loss over 24 months (with an initial phase of maximal weight loss from 1 to 6 months and a weight maintenance phase from 7 to 24 months) (17). Each participant provided written informed consent. An overview of these diets and sample menus are provided in Supplementary Tables 1 and 2. The low-fat, restricted-calorie diet was based on guidelines from the American Heart Association (18); participants assigned to this diet were counseled to consume low-fat grains, vegetables, fruits, and legumes and to limit their consumption of additional fats, sweets, and high-fat snacks (17). The Mediterranean, restricted-calorie diet was a moderate-fat diet rich in vegetables and low in red meat; olive oil and nuts were the main sources of added fat (17). The low-carbohydrate, non–restricted-calorie diet was adapted from the Atkins diet; participants started with an induction phase aimed to provide 20 g of carbohydrates per day for the first 2 months, which was gradually increased to a maximum of 120 g/day for the remainder of the 24-month period without limitation in total calories, protein, or fat. Participants were also counseled to choose vegetarian sources of fat and protein as well as to avoid trans fats (17). We present the effects of weight loss on SU concentrations at 6 months (i.e., maximal weight loss phase) as well as 24 months (capturing both the maximal weight loss and the maintenance phases) (17).
Participants
In the original study, men and women ages 40–65 years with a BMI of at least 27 kg/m2 or a diagnosis of either type 2 diabetes or coronary heart disease (regardless of age or BMI) were recruited into the trial (17). Individuals were excluded if they were pregnant or lactating, had a serum creatinine concentration of ≥177 μmol/L (2 mg/dL), liver dysfunction (increase by a factor of at least two above the upper limit of normal in ALT and AST concentrations), gastrointestinal problems that would prevent them from following any of the study diets, active cancer, or were participating in another trial. In total, 322 individuals were randomly assigned to one of the three diets over the study period and followed for weight loss. As shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Supplementary Fig. 1), this secondary study includes data from 235 participants who had stored blood samples available at both baseline and follow-up for SU measurement. This study was granted exempt status by the Partners HealthCare institutional review board (protocol identifier: 2016P002363).
Measurement of SU
Blood samples were obtained by venipuncture at 8:00 a.m. after a 12-h fast throughout the trial and stored at −80°C for future assay. In 2017, we used these samples to measure SU concentrations at baseline and at 6 and 24 months, using a standard automated uricase enzymatic assay on the Roche Modular P system (Roche Diagnostics, Indianapolis, IN).
Other Covariate Measurements and Definitions
Additional covariates were ascertained using medical history, questionnaires, laboratory specimens, and a physical examination, as described previously (17). Weight loss was the primary end point of the original DIRECT study. Weight measurements (without shoes) were obtained monthly and recorded to the nearest 0.1 kg. Height measurements were obtained at baseline using a wall-mounted stadiometer and recorded to the nearest millimeter. BMI was calculated for each participant as body mass divided by the square of their height in meters (kg/m2). Blood pressure was measured using an automated system following 5 min of rest. Blood samples were obtained by venipuncture as described above. Estimated glomerular filtration rate (eGFR) was calculated using the MDRD equation.
Statistical Analysis
We described baseline characteristics using mean ± SD and proportions. The primary outcome of this secondary analysis was the change in SU concentrations from baseline at 6 months (maximal weight loss phase) as well as 24 months (encompassing the maximal weight loss and maintenance phases) within the three diet groups. We assessed the within-person changes from baseline in each diet group with the use of a paired t test. We additionally focused on a prespecified and gout-risk relevant subgroup of individuals with hyperuricemia (defined as SU ≥416 μmol/L [>7 mg/dL]) at baseline, as was done in an ancillary study of the Dietary Approaches to Stop Hypertension (DASH) diet trial (19). For a mean ± SD difference between groups of at least 95 ± 95 μmol/L (1.6 ± 1.6 mg/dL) of SU concentration, with 17 participants per group and a type I error of 5%, the power to detect significant differences in SU is >80%. The corresponding difference between groups for the entire study sample will be 45 μmol/L (0.75 mg/dL), with 74 participants per group. The between-group differences between SU at baseline and 6 and 24 months were compared using a multivariable linear regression model that adjusted for baseline SU concentration. To evaluate the repeated blood measurements over time, we used generalized estimating equations to account for the nonindependence of the same bioindicator in the same individual over time. Interaction terms were added to assess changes over time by diet group. Finally, we used linear regression models to examine purported mediators for SU change at 24 months as a continuous variable. On the basis of available prior biologic knowledge (1,16,20), the mediators of interest were weight loss and change in fasting plasma insulin concentration at 24 months, while covariates included age, sex, diet group, and baseline SU. We restricted our analysis of the impact of fasting plasma insulin change to participants without diabetes (n = 201) because insulin concentration is affected by diabetes or antidiabetic medications. All analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC).
Results
Baseline Characteristics
In total, 235 trial participants had complete SU data and were included in the current analysis. Baseline characteristics were well balanced among the three diet groups (P for difference between groups > 0.05) (Table 1). The mean age in our study population was 52 years, the mean BMI was 30.5 kg/m2, and 88% of participants were men. The mean SU concentration at baseline was 363 μmol/L (6.1 mg/dL), and 24% of participants had SU ≥416 μmol/L (>7 mg/dL). Hypertension was present at baseline in 59% of participants, diabetes in 15%, and coronary heart disease in 36%. The 87 original participants who did not have blood samples available for SU measurements tended to have a higher BMI and weight and slightly worse blood pressure and metabolic parameters (Supplementary Table 3).
Table 1.
Baseline characteristics of study participants
| Baseline characteristic | Low-fat diet (n = 85) | Mediterranean diet (n = 76) | Low-carbohydrate diet (n = 74) |
|---|---|---|---|
| Intervention goal | ≤1,500 kcal/day for women and 1,800 kcal/day for men (≤30% calories from fat) | ≤1,500 kcal/day for women and 1,800 kcal/day for men (≤35% calories from fat) | No calorie restrictions (carbohydrate intake 20 g [induction] to ≤120 g [maintenance] daily) |
| Age (years) | 50.8 ± 6.4 | 52.7 ± 6.3 | 51.2 ± 6.2 |
| Male sex | 73 (85.7) | 66 (86.8) | 67 (90.5) |
| SU, μmol/L (mg/dL) | 369 ± 77 (6.2 ± 1.3) | 357 ± 89 (6.0 ± 1.5) | 357 ± 89 (6.0 ± 1.5) |
| SU ≥416 μmol/L (≥7 mg/dL) | 22 (25.9) | 17 (22.4) | 18 (24.3) |
| BMI (kg/m2) | 30.5 ± 3.1 | 30.1 ± 3.2 | 30.8 ± 3.5 |
| Weight (kg) | 90.7 ± 12.2 | 87.6 ± 12.1 | 91.5 ± 14.6 |
| Diabetes | 11 (12.9) | 11 (14.5) | 12 (16.2) |
| Coronary heart disease | 28 (32.9) | 36 (47.4) | 21 (28.4) |
| Blood pressure (mmHg) | |||
| Systolic | 129.8 ± 12.5 | 131.3 ± 16.3 | 129.4 ± 14.2 |
| Diastolic | 79.0 ± 8.8 | 80.0 ± 10.2 | 77.7 ± 8.1 |
| Lipid profile | |||
| HDL (mmol/L) | 0.99 ± 0.25 | 1.04 ± 0.25 | 0.97 ± 0.23 |
| LDL (mmol/L) | 2.98 ± 0.89 | 3.18 ± 0.89 | 3.13 ± 0.84 |
| Triglycerides (mmol/L) | 1.77 ± 0.71 | 1.89 ± 0.74 | 1.94 ± 0.95 |
| Total cholesterol:HDL-C ratio | 5.2 ± 1.4 | 5.2 ± 1.4 | 5.6 ± 1.5 |
| eGFR (mL/min/1.73 m2) | 96.4 ± 23.4 | 92.5 ± 18.6 | 99.4 ± 21.8 |
| Fasting plasma glucose (mmol/L) | 4.8 ± 1.1 | 5.3 ± 2.3 | 5.0 ± 1.2 |
| Fasting plasma glucose 5.6–6.9 mmol/L* | 3 (4.1) | 9 (13.8) | 5 (8.1) |
| Fasting plasma insulin (pmol/L) | 90.4 ± 50.0 | 96.6 ± 50.7 | 100.8 ± 77.1 |
| HOMA-IR | 2.8 ± 1.9 | 3.4 ± 2.7 | 3.3 ± 3.2 |
| HOMA-IR >3.0* | 26 (35.1) | 28 (43.1) | 25 (40.3) |
| Statins | 21 (24.7) | 20 (26.3) | 11 (15.0) |
| Antihypertensive therapy** | 19 (22.4) | 27 (35.5) | 26 (35.2) |
| Diuretics | 5 (5.9) | 10 (13.2) | 3 (4.0) |
Data are mean ± SD or n (%) unless otherwise indicated. HOMA-IR, HOMA of insulin resistance.
Among participants without diabetes.
Including diuretics.
SU Reduction During Maximal Weight Loss and Maintenance Periods
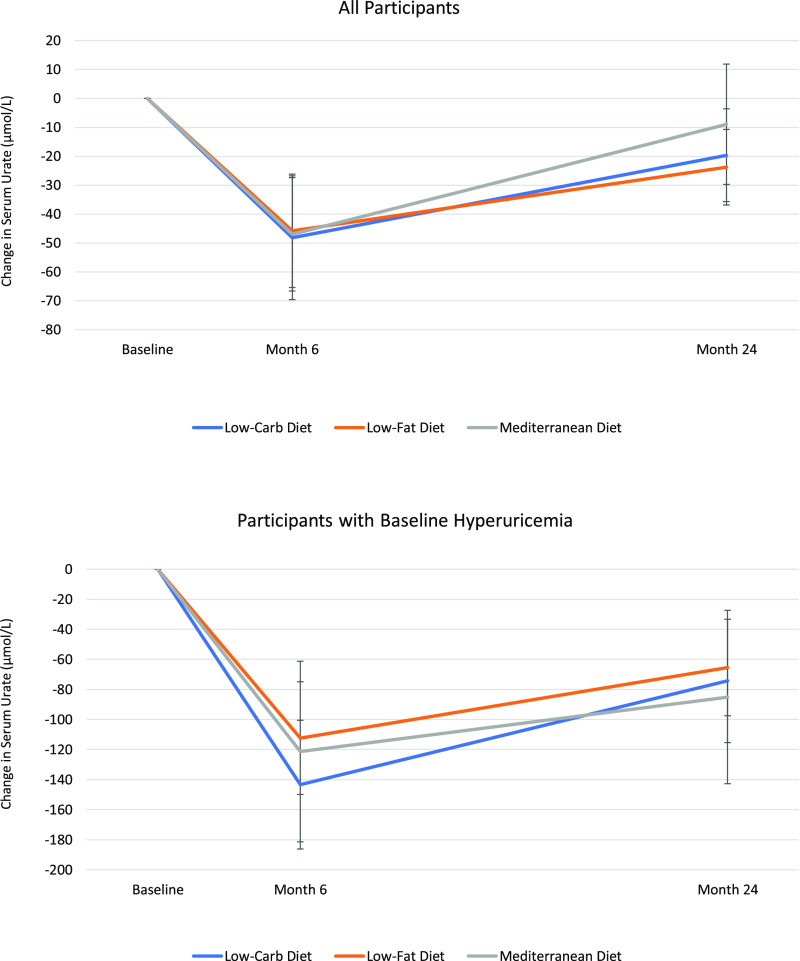
All three diet groups demonstrated a decrease in SU over the initial 6-month maximal weight loss period; the mean changes in SU from baseline to 6 months were −48 μmol/L (95% CI, −65 to −24) for the low-fat diet, −48 μmol/L (−65 to −30) for the Mediterranean diet, and −48 μmol/L (−71 to −30) for the low-carbohydrate diet (all P for within-group comparison < 0.001; P > 0.05 for between-group comparisons) (Fig. 1). During the entire 24-month period of the study, which included the weight maintenance phase over months 7–24, mean changes in SU from baseline were −24 μmol/L (−36 to −12) for the low-fat diet, −12 μmol/L (−30 to 12) for the Mediterranean diet, and −18 μmol/L (−36 to −6) for the low-carbohydrate diet (P = 0.38 for the interaction between diet group and time overall). There was no difference in the SU change among the three diet groups (P > 0.05 for between-group comparisons). Our analyses that excluded those on diuretics at baseline or adjusted for their use did not make a material difference in our results.
Figure 1.
SU response according to diet group, among all participants and among those with baseline hyperuricemia. Vertical bars indicate SEs. Low-Carb, low carbohydrate.
SU Reduction in Individuals With Hyperuricemia
Mean change in SU over these first 6 months was −113 μmol/L (95% CI, −149 to −77) for the low-fat diet, −119 μmol/L (−184 to −60) for the Mediterranean diet, and −143 μmol/L (−184 to −101) for the low-carbohydrate diet (all P for within-group comparison < 0.001; P > 0.05 for between-group comparisons) (Fig. 1 and Supplementary Fig. 2). The mean changes in SU from baseline to 24 months were −65 μmol/L (−95 to −36) for the low-fat diet, −83 μmol/L (−143 to −30) for the Mediterranean diet, and −77 μmol/L (−119 to 36) for the low-carbohydrate diet (all P for within-group comparison < 0.01; P > 0.05 for between-group comparisons) (Fig. 1). Our analyses that excluded those on diuretics at baseline or adjusted for their use did not make a material difference in our results.
Change in Cardiometabolic Risk Factors
At both 6 and 24 months, all three diets resulted in improvements in adiposity, lipid profile, systolic blood pressure, and fasting plasma insulin concentration compared with baseline (Fig. 2). The mean weight change at 6 months and 24 months, respectively, was −5.0 kg (95% CI, −5.8 to −4.0) and −3.1 kg (−4.0 to −2.2) for the low-fat diet, −5.1 kg (−6.2 to −4.1) and −4.5 kg (−5.9 to −3.2) for the Mediterranean diet, and −7.2 kg (−8.9 to −5.5) and −5.2 kg (−6.8 to −3.5) for the low-carbohydrate diet (all P for within-group comparison < 0.001). The difference in weight loss between the low-fat and low-carbohydrate diets between baseline and 6 months was significant (−2.1 kg, P = 0.02); no between-group differences in weight loss were observed at 24 months (all P > 0.05).
Figure 2.
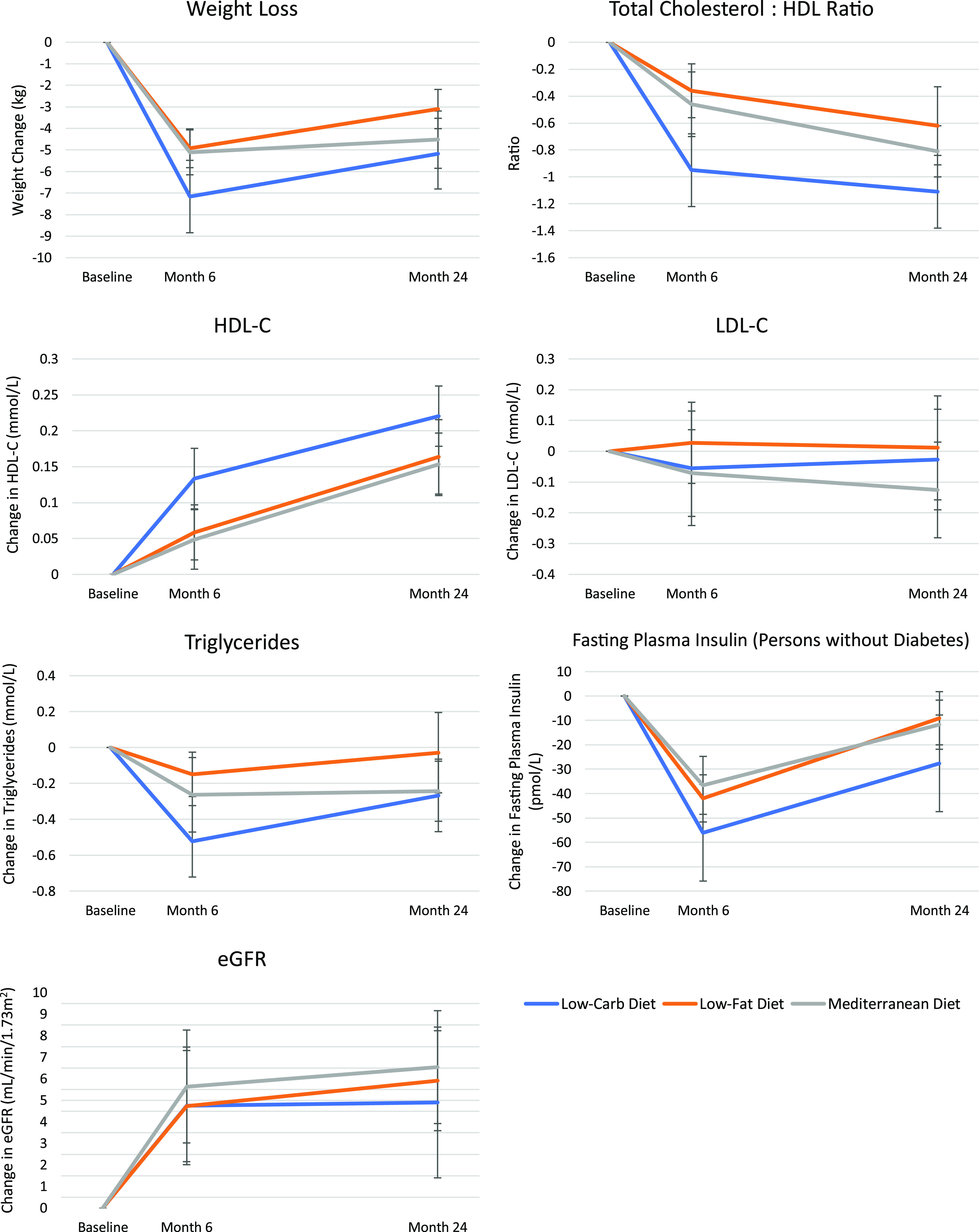
Change in cardiometabolic parameters at 6 and 24 months. Vertical bars indicate SEs. Low-Carb, low carbohydrate.
Both HDL cholesterol (HDL-C) concentration and the total cholesterol:HDL-C ratio improved significantly within each diet group both during the first 6 months and at 24 months (P < 0.05 for HDL-C; P < 0.001 for total cholesterol:HDL-C ratio) (Fig. 2). There were significant reductions in systolic blood pressure for all diet groups at both 6 months and 24 months (all P < 0.05 for within-group comparisons), and significant reductions in diastolic blood pressure for the Mediterranean diet group (P < 0.05 at both time points). Significant improvements in serum triglycerides were observed during the weight loss phase of the study in all three groups (all P for within-group comparison < 0.05), although at 24 months, the change was significant only in the Mediterranean and low-carbohydrate diet groups (Fig. 2). Similarly, significant improvements in fasting plasma insulin concentrations (among participants without diabetes) were observed in all three diet groups during the initial 6-month maximal weight loss period (P < 0.001), but at 24 months, these remained significant only for the Mediterranean and low-carbohydrate diet groups (Fig. 2). However, there were no significant between-group differences in fasting plasma insulin at either time point. eGFR improved in all groups similarly (all P for within-group comparison < 0.01; P > 0.05 for between-group comparisons).
Change in Cardiometabolic Parameters Among Participants With Baseline Hyperuricemia
Overall, similar improvements in cardiometabolic parameters were also observed when restricting to those with baseline hyperuricemia (SU ≥416 μmol/L [≥7 mg/dL]) (Supplementary Fig. 3). In the low-fat diet group, there were significant improvements between baseline and 6 months in weight (−5.8 kg, P < 0.001) and fasting plasma insulin (−39.6 pmol/L, P < 0.001) and between baseline and 24 months in weight (−3.2 kg, P = 0.002) and HDL-C (0.16 mmol/L, P = 0.001). In the Mediterranean diet group, there were significant improvements in weight, total cholesterol:HDL-C ratio, triglycerides, and fasting plasma insulin at both 6 and 24 months (all P for within-group comparison < 0.05); improvement in HDL-C was significant at 24 months only (P = 0.001). In the low-carbohydrate group, there were improvements in weight, total cholesterol:HDL-C ratio, HDL-C, and triglycerides at both 6 and 24 months (all P for within-group comparison < 0.01); improvement in fasting plasma insulin was only significant at 6 months (−38.9 pmol/L, P < 0.001). For between-group differences among those with baseline hyperuricemia, there were larger improvements in total cholesterol:HDL-C ratio (−0.79, P = 0.02), HDL-C (0.11 mmol/L, P = 0.03), and triglycerides (−0.47 mmol/L, P = 0.001) at 6 months in the low-carbohydrate group compared with the low-fat group.
Relation Between Change in Weight and Fasting Plasma Insulin Concentration and SU Reduction
We conducted multivariable linear regression analyses adjusting for age, sex, diet group, and baseline SU to examine purported mediators of change in SU concentrations over 24 months (Table 2). Weight loss at 24 months was associated with SU reduction in our univariable analysis (β = 0.05, P < 0.001) and remained significant in our multivariable analysis (β = 0.04, P < 0.001) (Table 2). Among trial participants without diabetes (n = 201), both change in fasting plasma insulin and weight loss at 24 months were significantly associated with SU reduction at 24 months (P = 0.03 for both) (Table 2). Both purported mediators remained significant after additional adjustment for eGFR at baseline or change (P < 0.05).
Table 2.
Predictors of reduction in SU at 24 months: multivariable regression model
| All (n = 235) | Participants without diabetes* (n = 201) | |||
|---|---|---|---|---|
| Variable in the models | β | P value | β | P value |
| Baseline SU, μmol/L (mg/dL) | −28.6 (−0.48) | <0.001 | −29.7 (−0.50) | <0.001 |
| Weight loss at 24 months (kg) | 0.04 | <0.001 | 0.03 | 0.03 |
| Fasting plasma insulin reduction at 24 months (pmol/L) | NA | NA | 0.21 | 0.21 |
| Age (years) | −0.01 | 0.47 | 0.002 | 0.85 |
| Male sex | 0.66 | 0.001 | 0.82 | <0.001 |
| Mediterranean diet vs. low-fat assigned diet | 0.15 | 0.32 | 0.16 | 0.32 |
| Low-carbohydrate vs. low-fat assigned diet | 0.03 | 0.87 | 0.02 | 0.88 |
NA, not applicable.
Limited to participants without diabetes because insulin concentration is affected by diabetes or antidiabetic medications.
Conclusions
In this secondary analysis of DIRECT, we found that all three weight loss diets, including the low-carbohydrate, high-protein diet, lowered SU concentrations similarly. The SU reduction among those with hyperuricemia was large, ranging from 113 to 143 μmol/L (1.9–2.4 mg/dL) over the first 6 months. Although attenuated by 24 months, the SU reduction remained substantial, ranging from 65 to 83 μmol/L (1.1–1.4 mg/dL). Our analyses support that the observed SU reductions are mediated through reduction in weight and insulin resistance, as in previous experimental studies, further strengthening the notion of hyperuricemia as a part of the insulin resistance syndrome (1,2) or prediabetes. Accordingly, other cardiovascular risk factors also improved in all diet groups, including HDL-C, triglycerides, total cholesterol:HDL-C ratio, systolic blood pressure, and fasting plasma insulin concentrations, while such benefits remain controversial with effective urate-lowering drugs to date (9–12).
These cardiometabolic benefits are particularly relevant for individuals with hyperuricemia and gout given their high level of cardiometabolic morbidity and persistent premature mortality gap in recent decades (3,4,21), with no apparent benefits of urate-lowering drugs on these outcomes in large-scale trials to date (9–11). The cardiometabolic benefits of the Mediterranean diet, which consists of a high intake of monounsaturated fat, plant proteins, whole grains, and fish; moderate intake of alcohol; and low consumption of red meat, refined grains, and sweets, are well-established (22). For example, in the Prevention with Mediterranean Diet (PREDIMED) trial, which included 7,447 individuals at high risk of cardiovascular disease, two variations of the Mediterranean diet, one supplemented with extra virgin olive oil and the other supplemented with nuts, resulted in 31% and 28% reductions in the risk of major cardiovascular events, respectively (23), and led to a >50% lower risk of incident type 2 diabetes compared with the control diet (24). The Lyon Diet Heart Trial, which enrolled 605 patients with history of myocardial infarction, found that a Mediterranean diet led to a 73% lower rate of coronary events and a 70% lower rate of total mortality compared with a usual postinfarct “prudent” diet (25). Although not specifically included in this trial, a similarly structured cardiovascular diet is the DASH diet, which is rich in fruits, vegetables, and low-fat dairy products and is low in animal protein but recommends a considerable amount of plant protein from legumes and nuts (18). The DASH diet reduces blood pressure (26,27) as well as total cholesterol and LDL-C (26,27) and has been associated with a lower risk of cardiovascular disease (28–30), type 2 diabetes (31,32), and mortality (33,34). Furthermore, similar to our results, the DASH diet was found to reduce SU concentrations (19) and the risk of gout (6).
The SU-lowering effect of the low-carbohydrate, high-protein diet (i.e., the opposite of the conventional low-purine approach for gout) is counterintuitive; however, the present work expands on a previous open-label trial of 13 patients with gout that aimed to lower weight and insulin resistance by reducing calories and carbohydrate intake with a proportional increase in protein intake (20). Over 16 weeks, this diet led to a 95 μmol/L (1.6 mg/dL) reduction in SU overall and decrease in gout flare frequency from 2.1 to 0.6 per month. Although DIRECT was not conducted among patients with gout, the effect size of SU decline in the study participants with hyperuricemia assigned to the low-carbohydrate diet was comparable to that seen among the patients with gout in the open-label trial. That trial of patients with gout also resulted in a weight loss of 7.7 kg as well as improvements in total cholesterol, LDL-C, cholesterol ratio, and triglyceride concentrations (20), similar to our findings. Collectively, these findings suggest that simultaneous improvements in SU and cardiometabolic risk factors over 2 years are also possible with the low-carbohydrate, high-protein diet, contrary to the popular belief for the need to reduce protein intake. These findings also support the notion that weight loss through calorie restriction is just as important a mediator of SU reduction as diet composition or quality.
The fact that all three diets resulted in similar improvements in SU and multiple cardiometabolic risk factors at both 6 and 24 months in our study is meaningful because it suggests that there are multiple viable dietary options for patients looking to simultaneously improve SU concentrations and their cardiometabolic health. The choice can be guided by the individual’s concurrent cardiometabolic comorbidity profiles; personal preferences should also be considered to help to maximize adherence (17). To that end, an additional 4-year follow-up of DIRECT (i.e., total 6 years) suggested that the Mediterranean diet may have the best adherence and therefore sustained weight loss among the three diets (35). Further studies are needed to evaluate these strategies to personalize these dietary and lifestyle recommendations on the basis of comorbidities, preferences, and sustainability factors for precision medicine in hyperuricemia and gout care.
We found that weight loss and fasting plasma insulin reduction both significantly mediate SU improvement, consistent with prior reports. Previous studies have consistently found strong associations among fasting plasma insulin concentrations, insulin resistance, prediabetes, and SU concentrations (1,16,36,37). For example, the prevalence of prediabetes or diabetes in the National Health and Nutrition Examination Survey (NHANES III) data increased in a dose-response fashion from 23 to 37%, 39%, and 54% for SU levels <357, 357–470, 476–589, and ≥595 μmol/L, respectively (37). Obesity also increases urate concentrations through decreased renal urate excretion (by increasing insulin resistance) (1,38,39) and increased urate production (2,8). Exogenous insulin was shown to reduce the renal excretion of urate in both healthy subjects and those with hypertension (16,40). Insulin may enhance renal urate reabsorption through stimulation of GLUT9 or other renal urate transporters. Collectively, these findings further the notion of hyperuricemia as a part of the insulin resistance syndrome (1,2) or prediabetes; therapeutic measures for the latter (weight loss and diets) will likely be central for hyperuricemia as well.
The main strength of our study is the use of data from a randomized dietary intervention trial of three diets to study the impact of weight loss (during both the intensive weight loss and the weight maintenance phases) on SU concentrations and cardiovascular risk factors simultaneously. The potential limitations of our study also deserve comment. First, the trial population had a high proportion of males, elevated baseline SU concentrations, and high prevalence of cardiometabolic comorbidities at baseline, sharing characteristics with that of a gout population. A quarter of participants had baseline hyperuricemia (SU ≥416 μmol/L [≥7 mg/dL]), also comparable to that of patients with gout. These characteristics are known risk factors for developing gout, and thus, our study results would be directly generalizable to those populations at heightened risk of developing gout. Nevertheless, these results warrant confirmation among a cohort of patients with gout, including the ascertainment of secondary outcomes, such as gout flares. If findings of a similar magnitude observed in individuals with hyperuricemia can be replicated in patients with gout, these dietary interventions carry the potential for clinically meaningful improvements in SU and attainment of target SU concentrations (e.g., <357 μmol/L [<6 mg/dL]) (Supplementary Fig. 2), particularly for those with moderately high SU concentrations. Second, the trial’s end points were weight loss and established cardiovascular risk factors rather than hard end points, thus calling for confirmation of such benefits in future studies. Third, while the current secondary analysis is based on another well-performed trial, our findings, particularly the subgroup data, ideally should be replicated in further trials. Fourth, the unique nature of the workplace in this study allowed a closely monitored dietary intervention for 2 years but may render difficulties generalizing the results to other populations. However, similar strategies to maintain adherence are likely to be applicable elsewhere. Fifth, because our low-carbohydrate diet group was encouraged to consume a variety of protein and fat sources, including vegetarian sources, while avoiding trans fats, our findings may not apply to the effects of a more liberal ketogenic diet without this type of counseling. Sixth, we had fasting plasma glucose and insulin levels but not HbA1c, precluding a subgroup analysis of prediabetes, which is relevant to the development of hyperuricemia. Finally, as observed in this trial, weight maintenance in real-world settings is an ongoing challenge and a key topic of ongoing and future research areas.
In conclusion, we found that low-fat, restricted-calorie; Mediterranean, restricted-calorie; and low-carbohydrate (high-protein), non–restricted-calorie diets can all lower SU concentrations, particularly among those with baseline hyperuricemia, although the overall effect size is modest compared with that of a typical urate-lowering drug. These effects were mediated by weight loss and changes in insulin concentrations, further strengthening the notion of hyperuricemia as a part of the insulin resistance syndrome (1,2) or prediabetes. Accordingly, cardiovascular risk factors improved consistently across these diets, whereas such benefits remain controversial with effective urate-lowering drugs. These findings support a beneficial role for these dietary interventions in improving both cardiovascular risk factors and SU control in hyperuricemia management and that there may be several viable dietary options available to patients, which can provide personalized medicine pathways to suit patient comorbidities and preferences for adherence.
Article Information
Acknowledgments. The authors thank the DIRECT participants for their consistent cooperation.
Funding. This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P50-AR-060772 and R01-AR-065944). C.Y. is supported by the National Institutes of Health Ruth L. Kirschstein Institutional National Research Service Award (T32-AR-007258) and the Rheumatology Research Foundation Scientist Development Award. N.M. is supported by a Canadian Institutes of Health Research Fellowship Award. S.K.R. is supported by a Canadian Institutes of Health Research Doctoral Foreign Study Award. G.C. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K24-DK-091417). I.S. is supported by Deutsche Forschungsgemeinschaft (SFB 1052) and the Israeli Science Foundation and Israel Ministry of Science and Technology.
Duality of Interest. G.C. reports research support from Decibel Therapeutics and consulting fees from AstraZeneca, Allena Pharmaceuticals, Shire/Takeda, Dicerna, and Orfan and is the chief medical officer at OM1, Inc. H.K.C. reports research support from Ironwood and Horizon and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.Y. was involved in the analysis and interpretation of the data and drafted the manuscript. N.M., S.K.R., N.L., G.C., and H.K.C. were involved in the analysis and interpretation of the data and revised the manuscript critically for important intellectual content. D.S. and I.S. were involved in the study design and acquisition, analysis, and interpretation of the data and revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript to be submitted for publication. H.K.C. is the guarantor of this work and, as such, had access to all the data in study and takes responsibility for integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a podium presentation at the American College of Rheumatology 2019 Annual Meeting, Atlanta, GA, 8–13 November 2019.
Footnotes
Clinical trial reg. no. NCT00160108, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.12780059.
N.M. and S.K.R. contributed equally to this work.
References
- 1.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991;266:3008–3011 [PubMed] [Google Scholar]
- 2.Fam AG. Gout, diet, and the insulin resistance syndrome. J Rheumatol 2002;29:1350–1355 [PubMed] [Google Scholar]
- 3.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med 2012;125:679–687.e1 [DOI] [PubMed] [Google Scholar]
- 4.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894–900 [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093–1103 [DOI] [PubMed] [Google Scholar]
- 6.Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK. The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ 2017;357:j1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005;165:742–748 [DOI] [PubMed] [Google Scholar]
- 8.Emmerson BT. The management of gout. N Engl J Med 1996;334:445–451 [DOI] [PubMed] [Google Scholar]
- 9.White WB, Saag KG, Becker MA, et al.; CARES Investigators . Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018;378:1200–1210 [DOI] [PubMed] [Google Scholar]
- 10.Becker MA, Schumacher HR Jr., Wortmann RL, et al. . Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–2461 [DOI] [PubMed] [Google Scholar]
- 11.Doherty M, Jenkins W, Richardson H, et al. . Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doria A, Galecki A, Spino C, Mauer M. Preventing Early Renal Loss in Diabetes (PERL) study: outcome of a 3-year trial of serum uric acid reduction with allopurinol (Abstract). J Am Soc Nephrol 2019;30:B2 [Google Scholar]
- 13.Eckel RH, Jakicic JM, Ard JD, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2960–2984 [DOI] [PubMed] [Google Scholar]
- 14.Jang WC, Nam Y-H, Park S-M, et al. . T6092C polymorphism of SLC22A12 gene is associated with serum uric acid concentrations in Korean male subjects. Clin Chim Acta 2008;398:140–144 [DOI] [PubMed] [Google Scholar]
- 15.Quiñones Galvan A, Natali A, Baldi S, et al. . Effect of insulin on uric acid excretion in humans. Am J Physiol 1995;268:E1–E5 [DOI] [PubMed] [Google Scholar]
- 16.Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 1997;92:51–58 [DOI] [PubMed] [Google Scholar]
- 17.Shai I, Schwarzfuchs D, Henkin Y, et al.; Dietary Intervention Randomized Controlled Trial (DIRECT) Group . Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–241 [DOI] [PubMed] [Google Scholar]
- 18.Krauss RM, Eckel RH, Howard B, et al. . AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000;102:2284–2299 [DOI] [PubMed] [Google Scholar]
- 19.Juraschek SP, Gelber AC, Choi HK, Appel LJ, Miller ER III. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet and sodium intake on serum uric acid. Arthritis Rheumatol 2016;68:3002–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher MC, Rai SK, Lu N, Zhang Y, Choi HK. The unclosing premature mortality gap in gout: a general population-based study. Ann Rheum Dis 2017;76:1289–1294 [DOI] [PubMed] [Google Scholar]
- 22.Hu FB. The Mediterranean diet and mortality--olive oil and beyond. N Engl J Med 2003;348:2595–2596 [DOI] [PubMed] [Google Scholar]
- 23.Estruch R, Ros E, Salas-Salvadó J, et al.; PREDIMED Study Investigators . Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 24.Salas-Salvadó J, Bulló M, Babio N, et al.; PREDIMED Study Investigators . Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lorgeril M, Renaud S, Mamelle N, et al. . Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454–1459 [DOI] [PubMed] [Google Scholar]
- 26.Appel LJ, Moore TJ, Obarzanek E, et al.; DASH Collaborative Research Group . A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–1124 [DOI] [PubMed] [Google Scholar]
- 27.Sacks FM, Svetkey LP, Vollmer WM, et al.; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 28.Djoussé L, Ho YL, Nguyen XT, et al.; VA Million Veteran Program . DASH score and subsequent risk of coronary Artery disease: the findings from Million Veteran Program. J Am Heart Assoc 2018;7:e008089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. . Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation 2015;132:2212–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720 [DOI] [PubMed] [Google Scholar]
- 31.Liese AD, Nichols M, Sun X, D’Agostino RB Jr., Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care 2009;32:1434–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 2011;34:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. . Association of changes in diet quality with total and cause-specific mortality. N Engl J Med 2017;377:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh A, Lipsitz SR, Natarajan S. Association between a DASH-like diet and mortality in adults with hypertension: findings from a population-based follow-up study. Am J Hypertens 2009;22:409–416 [DOI] [PubMed] [Google Scholar]
- 35.Schwarzfuchs D, Golan R, Shai I. Four-year follow-up after two-year dietary interventions. N Engl J Med 2012;367:1373–1374 [DOI] [PubMed] [Google Scholar]
- 36.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol 1998;8:250–261 [DOI] [PubMed] [Google Scholar]
- 37.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 2007;120:442–447 [DOI] [PubMed] [Google Scholar]
- 38.Emmerson BT. Alteration of urate metabolism by weight reduction. Aust N Z J Med 1973;3:410–412 [DOI] [PubMed] [Google Scholar]
- 39.Yamashita S, Matsuzawa Y, Tokunaga K, Fujioka S, Tarui S. Studies on the impaired metabolism of uric acid in obese subjects: marked reduction of renal urate excretion and its improvement by a low-calorie diet. Int J Obes 1986;10:255–264 [PubMed] [Google Scholar]
- 40.Muscelli E, Natali A, Bianchi S, et al. . Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens 1996;9:746–752 [DOI] [PubMed] [Google Scholar]