Abstract
OBJECTIVE
Bariatric surgery is associated with diabetes remission and prevention of diabetes-related complications. The ABCD, DiaRem, Ad-DiaRem, DiaBetter, and individualized metabolic surgery scores were developed to predict short- to medium-term diabetes remission after bariatric surgery. However, they have not been tested for predicting durable remission nor the risk of diabetes complications, nor compared with diabetes duration alone.
RESEARCH DESIGN AND METHODS
We identified 363 individuals from the surgically treated group in the prospective Swedish Obese Subjects study with preoperative type 2 diabetes and for whom data (preoperative age, BMI, C-peptide, HbA1c, oral diabetes medications, insulin use, and diabetes duration) were available for calculation of remission scores. Partial remission (after 2 and 10 years) was defined as blood glucose <6.1 mmol/L or HbA1c <6.5% (48 mmol/mol) and no diabetes medication. Information on diabetes complications (at baseline and over 15 years of follow-up) was obtained from national health registers. Discrimination was evaluated by area under receiver operating characteristic curves (AUROCs).
RESULTS
For 2-year diabetes remission, AUROCs were between 0.79 and 0.88 for remission scores and 0.84 for diabetes duration alone. After 10 years, the predictive ability of scores decreased markedly (AUROCs between 0.70 and 0.76), and no score had higher predictive capacity than diabetes duration alone (AUROC = 0.73). For development of microvascular and macrovascular diabetes complications over 15 years, AUROCs for remission scores were 0.70–0.80 and 0.62–0.71, respectively, and AUROCs for diabetes duration alone were 0.77 and 0.66, respectively.
CONCLUSIONS
Remission scores and diabetes duration are good predictors of short-term diabetes remission. However, for durable remission and risk of complications, remission scores and diabetes duration alone have limited predictive ability.
Introduction
Type 2 diabetes is a progressive disease associated with severe macrovascular complications (e.g., myocardial infarction, stroke, peripheral vascular disease) and microvascular complications (affecting eyes, nerves, and kidneys) (1). Diabetes remission is common after bariatric surgery, and randomized controlled trials have shown that bariatric surgery is superior to medical treatment for achieving diabetes remission in the short term (2). Data from the Swedish Obese Subjects (SOS) study have shown that a short duration of diabetes before surgery is highly associated both with a higher diabetes remission rate at 2 years and a lower risk for diabetes relapse between 2 and 10 years of follow-up (3). Moreover, patients in the SOS study with a diabetes duration of <1 year had the highest chance of remission and lowest risk of microvascular complications (3). Diabetes duration has been identified as an independent predictor in several other studies (4–12), and diabetes duration cutoffs of 2–10 years have been suggested to best identify patients having the highest chance of diabetes remission (4,9,11,13–15).
Several different scoring models intended as clinical tools for prediction of diabetes remission after bariatric surgery have been suggested. These include the ABCD (6); DiaRem (16) and the related scores Ad-DiaRem (17) and DiaBetter (18), which are all based on retrospective data from patients who have undergone gastric bypass (GBP); and the individualized metabolic surgery (IMS) score developed to better optimize choice of surgical procedure between GBP and sleeve gastrectomy (5). All five scores use various combinations of preoperative variables that were found to be significantly associated with short- to medium-term diabetes remission, depending on the data available for model development; ABCD, Ad-DiaRem, DiaBetter, and IMS, but not DiaRem, include diabetes duration as a predictor variable. Earlier studies have tested the validity of these scoring models for predicting short- to medium-term diabetes remission (i.e., 2–5 years) (5,13,18–24), but no studies have directly compared the scoring systems with respect to accuracy for durable remission (10 years) or the risk of diabetes complications. In addition, no studies have compared the discriminative capacity of these scoring models with diabetes duration as a single predictor.
The objective of this report was to test the predictive capacity of the ABCD, DiaRem, Ad-DiaRem, DiaBetter, and IMS scores and diabetes duration alone for prediction of durable diabetes remission and development of microvascular and macrovascular complications.
Research Design and Methods
Study Design and Participants
The ongoing, prospective controlled SOS intervention study enrolled 4,047 individuals with obesity between 1987 and 2001, as previously described (3,25,26) (Supplementary Material). In brief, 2010 individuals chose to undergo surgery, and a contemporaneously matched obese control group of 2,037 participants was created using 18 variables. The surgery and control groups had identical inclusion and exclusion criteria. The inclusion criteria were age 37–60 years and a BMI of ≥34 kg/m2 in men and ≥38 kg/m2 in women before or at the time of the matching examination. The exclusion criteria were established to exclude patients with unacceptable surgical risks. Diabetes remission and diabetes complications were prespecified secondary end points of the SOS study.
Patients were followed with physical examinations and questionnaires (which included questions about diabetes medication and diabetes duration) at baseline (4 weeks before the start of the intervention) and 0.5, 1, 2, 3, 4, 6, 8, and 10 years after study start. At the baseline examination and the 2- and 10-year follow-ups, blood samples were taken after an overnight fast. HbA1c and C-peptide levels were analyzed at the St. Vincent’s Healthcare Group, Dublin, Ireland, accredited by Irish National Accreditation Board: Registration number 192MT, in compliance with ISO 15189:2012. All other measurements were analyzed at the Central Laboratory, Sahlgrenska University Hospital, Gothenburg, Sweden, accredited according to International Organization for Standardization/International Electrochemical Commission 15189:2007 standards. The study was approved by the relevant ethical review boards, and written or oral informed consent was obtained from all participants.
Diabetes-Related Definitions
Diabetes status was determined at baseline and at the 2- and 10-year follow-ups. Type 2 diabetes was defined as HbA1c ≥ 6.5% (≥48 mmol/mol) or fasting blood glucose ≥6.1 mmol/L (corresponding to a fasting plasma glucose of ≥7 mmol/L) or by diabetes medication use (insulin, oral antidiabetic drugs, or both). Partial diabetes remission was defined as HbA1c <48 mmol/mol or fasting blood glucose <6.1 mmol/L (plasma glucose <7.0 mmol/L) without receipt of diabetes medication. Complete diabetes remission was defined as HbA1c <5.7% (<39 mmol/mol) or fasting blood glucose <5.0 mmol/L (plasma glucose <5.6 mmol/L) (27).
From 1987 through 2009, fasting glucose concentrations were measured in venous whole blood. After 2009, venous fasting plasma glucose was measured, and the concentrations were converted to those for blood glucose (25). The study was initiated before repeated measurements were routinely used for the diagnosis of type 2 diabetes; therefore, single determinations of fasting glucose and HbA1c were used. For patients with onset of diabetes before age 35 years, type 1 diabetes and latent autoimmune diabetes of adults were ruled out by excluding patients positive for GAD or islet cell antibodies or with C-peptide values <1.11 ng/mL at baseline; two patients were excluded (26).
Scoring Systems
The ABCD, DiaRem, Ad-DiaRem, DiaBetter, and IMS scores were calculated using preoperative variables as previously reported (5,6,16–18) (Supplementary Table 1). The ABCD score is based on age, BMI, C-peptide, and diabetes duration. The DiaRem score is based on age, HbA1c, and the use of metformin, sulfonylureas, glitazones, and/or insulin. The Ad-DiaRem includes the DiaRem criteria together with the number of antidiabetic drugs (all currently clinically available drug classes used for type 2 diabetes treatment) and diabetes duration. The DiaBetter score is based on HbA1c, use of metformin, other antidiabetic drugs, and diabetes duration. The IMS score is based on HbA1c, insulin treatment, number of antidiabetic drugs, and diabetes duration. For all scoring systems apart from the ABCD score, lower scores should predict a high chance of diabetes remission, and higher scores should predict a low chance of diabetes remission.
Diabetes Complications
Microvascular and macrovascular diabetes complications requiring hospital or specialist outpatient treatment or that were associated with death during follow-up were traced by searching the Swedish National Patient Register and the Swedish Cause of Death Register until 31 December 2016 (3). The inpatient components of the National Patient Register and the Cause of Death Register have 99% coverage. For specialist outpatient care, the National Patient Register coverage for somatic diseases has been assessed as 78% (28). Validation studies have shown that 80–90% of the diagnoses in the National Patient Register are accurate (29,30). ICD-9 and ICD-10 and intervention codes are listed in Supplementary Table 2.
Statistical Analyses
Mean values, SDs, and percentages were used to describe the baseline characteristics of the participants. Differences between those in remission versus nonremission were tested with t tests for continuous variables and Fisher exact test for dichotomous variables.
We assessed performance of the ABCD, DiaRem, DiaBetter, Ad-DiaRem, and IMS scores and diabetes duration alone using measures of discrimination (for all) and calibration (for DiaRem-related scores only) (31). Discrimination describes the ability of the predictor to distinguish those at high chance of diabetes remission or being free from diabetes complications from those having a low chance. It was evaluated by calculating the area under the receiver operating characteristic curves (AUROCs) for the different scores and diabetes duration alone. Calibration indicates the ability of a score to correctly predict the chance of remission and was performed using a χ2 goodness-of-fit test (31). In the calibration plot, the predicted remission rates in previously defined score groups (18,21) were plotted against the corresponding observed remission rates. If the predicted remission rate equals the observed remission rate throughout the entire score range, the calibration plot would then follow the 45° line. Youden index was used to determine the optimal cutoff for the AUROCs (i.e., the point at which the model would have maximal sum of sensitivity and specificity) (32).
In this study, data were analyzed per protocol (i.e., censored at time of reoperation if it resulted in change of treatment group). A two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata 15.1 software.
Results
Description of Baseline Characteristics and Follow-up Rates
The current analyses include 363 patients from the surgery group of the SOS study who had type 2 diabetes and data available for calculation of remission scores at baseline (Supplementary Fig. 1). In 194 (53.4%) of these patients, type 2 diabetes was diagnosed at baseline. In terms of surgical procedure, 234 (64.5%) underwent vertical banded gastroplasty (VBG), 64 (17.6%) underwent nonadjustable or adjustable banding, and 65 (17.9%) underwent GBP.
Preoperative baseline characteristics, in the whole cohort and divided by 2- and 10-year remission status, are presented in Table 1. Lower baseline levels of blood glucose and HbA1c, shorter baseline diabetes duration, and lower DiaRem, Ad-DiaRem, DiaBetter, and IMS scores were observed in patients who were in remission after both 2 and 10 years. Patients in remission at 2 and 10 years also had higher baseline ABCD scores, higher levels of C-peptide, and slightly higher BMI but similar baseline age and levels of insulin compared with the nonremission groups. The overall remission rate was 72% at 2 years and 35% at 10 years (Supplementary Fig. 1).
Table 1.
Preoperative characteristics of the whole cohort and the cohort stratified by 2- and 10-year remission status
| 2-year status (n = 307) | 10-year status (n = 249) | ||||||
|---|---|---|---|---|---|---|---|
| All | Remission | Nonremission | P value | Remission | Nonremission | P value | |
| Patients, n | 363 | 220 | 87 | 86 | 163 | ||
| Women | 217 (59.8) | 137 (62.3) | 49 (56.3) | 0.365 | 59 (68.6) | 92 (56.4) | 0.076 |
| Age (years) | 48.6 (6.0) | 48.7 (5.9) | 49.0 (6.0) | 0.633 | 48.3 (6.0) | 49.0 (5.6) | 0.360 |
| BMI (kg/m2) | 42.3 (4.9) | 42.8 (4.9) | 41.1 (3.8) | 0.004 | 43.6 (5.8) | 41.6 (4.2) | 0.002 |
| HbA1c | |||||||
| % | 7.8 (1.5) | 7.4 (1.4) | 8.7 (1.5) | <0.001 | 7.2 (1.3) | 8.1 (1.5) | <0.001 |
| mmol/mol | 61.3 (16.5) | 57.1 (15.0) | 71.7 (15.9) | <0.001 | 54.7 (14.1) | 64.5 (16.8) | <0.001 |
| Blood glucose (mmol/L) | 8.2 (2.8) | 7.5 (2.3) | 10.0 (2.9) | <0.001 | 7.2 (2.1) | 8.7 (2.9) | <0.001 |
| Insulin (mU/L) | 28.3 (19.3) | 27.9 (13.3) | 26.4 (17.4) | 0.411 | 29.6 (14.0) | 27.0 (22.2) | 0.327 |
| C-peptide (ng/mL) | 4.5 (1.5) | 4.8 (1.4) | 3.8 (1.5) | <0.001 | 4.8 (1.4) | 4.3 (1.4) | 0.004 |
| Diabetes duration at baseline (years) | 2.4 (4.2) | 0.9 (1.5) | 6.4 (6.2) | <0.001 | 0.5 (1.2) | 3.0 (4.2) | <0.001 |
| Screen-detected T2D at baseline | 194 (53.4) | 147 (66.8) | 13 (14.9) | <0.001 | 69 (80.2) | 64 (39.3) | <0.001 |
| Complications at baseline | |||||||
| Microvascular | 5 (1.4) | 2 (0.9) | 2 (2.3) | 0.319 | 1 (1.2) | 3 (1.8) | 1.000 |
| Macrovascular | 19 (5.2) | 5 (2.3) | 12 (13.8) | <0.001 | 0 (0) | 13 (8.0) | 0.005 |
| Remission scores at baseline | |||||||
| ABCD | 6.0 (1.7) | 6.5 (1.3) | 4.5 (1.9) | <0.001 | 6.8 (1.4) | 5.6 (1.7) | <0.001 |
| DiaRem | 6.3 (4.2) | 4.8 (3.0) | 10.3 (4.7) | <0.001 | 4.2 (2.6) | 7.4 (4.5) | <0.001 |
| Ad-DiaRem | 6.6 (4.0) | 5.2 (2.8) | 10.6 (4.2) | <0.001 | 4.7 (2.7) | 7.6 (4.0) | <0.001 |
| DiaBetter | 2.9 (2.5) | 1.9 (1.8) | 5.6 (2.4) | <0.001 | 1.5 (1.7) | 3.5 (2.6) | <0.001 |
| IMS | 29.2 (31.3) | 16.9 (19.5) | 62.3 (34.0) | <0.001 | 12.1 (16.2) | 36.7 (32.1) | <0.001 |
Data are mean (SD) or n (%) unless otherwise indicated. Missing data at baseline: blood glucose, n = 1; insulin, n = 3. T2D, type 2 diabetes.
There were no significant differences for baseline rates of microvascular diabetes complications, but higher baseline rates of macrovascular diabetes complications were observed in the nonremission groups (Table 1). The overall rates of microvascular and macrovascular complications over 15 years were 18% and 30%, respectively. Complication rates were markedly higher in the nonremission groups (Supplementary Fig. 1).
To enable comparison with previously reported remission rates for the scores (5,18,21,33), remission and complication rates in previously defined score groups are shown in Supplementary Fig. 2. Remission rates at 2 years were highest in patients with lower DiaRem, Ad-DiaRem, DiaBetter, and IMS scores and higher ABCD scores, in agreement with earlier reports (5,16–18,21,33). Overall, remission rates decreased at 10 years, and complication rates at 15 years increased with decreasing remission rates.
Preoperative Remission Scores and Diabetes Duration Alone as Predictors of Type 2 Diabetes Remission
The predictive capacities of the scores and diabetes duration alone for partial remission at 2 and 10 years are shown in Fig. 1. For partial diabetes remission at 2 years, the AUROC was 0.79 (95% CI 0.73–0.86) for ABCD, 0.86 (0.81–0.90) for DiaRem, 0.85 (0.80–0.90) for Ad-DiaRem, 0.88 (0.84–0.92) for DiaBetter, 0.88 (0.83–0.92) for IMS, and 0.84 (0.79–0.89) for diabetes duration alone (Fig. 1, left). The predictive capacity of diabetes duration alone was similar to the ABCD, DiaRem, and Ad-DiaRem scores (P for all comparisons not significant). However, at 2 years, both the DiaBetter and the IMS scores performed better than diabetes duration alone (P = 0.008 and P < 0.001, respectively).
Figure 1.
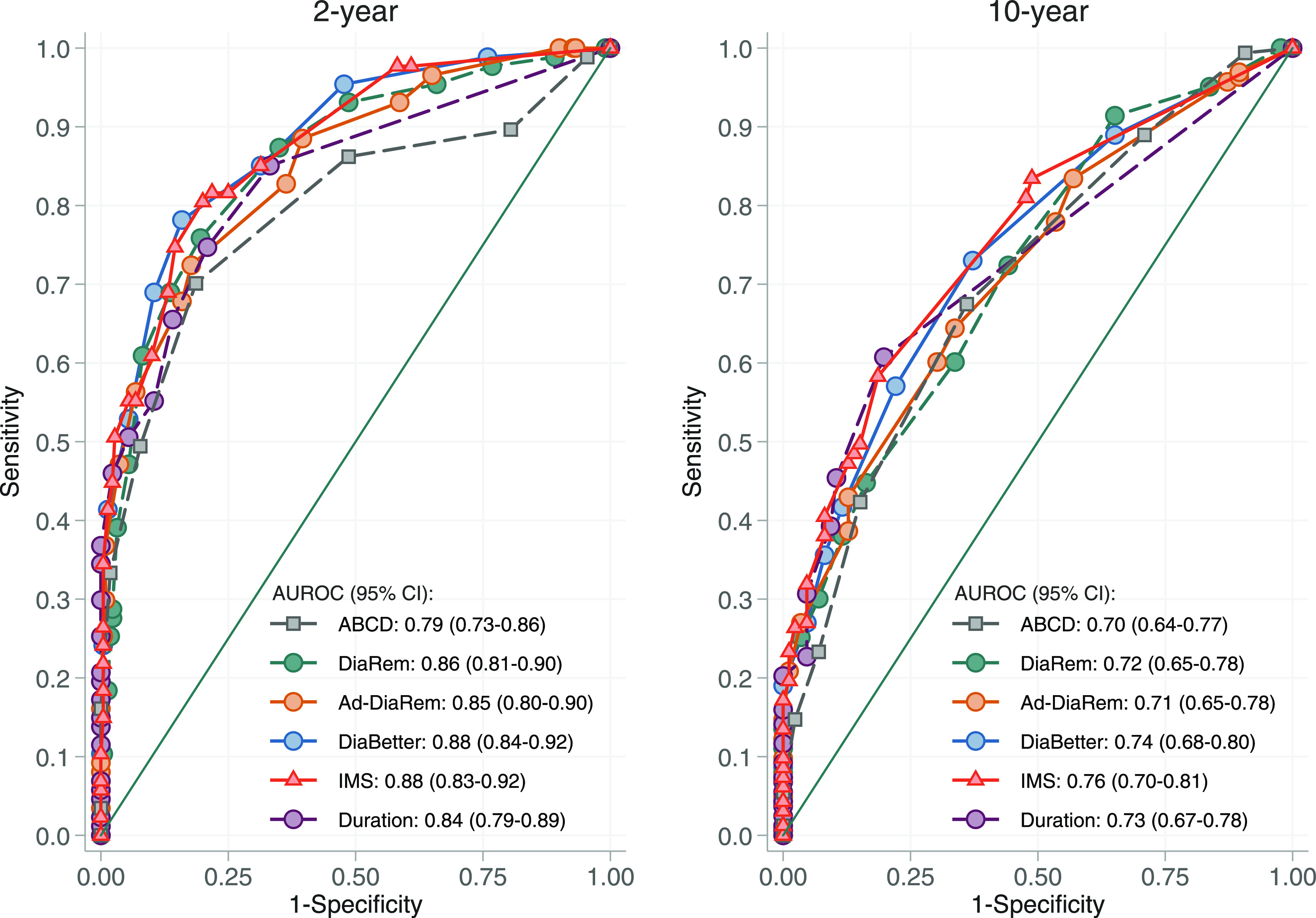
Comparison of the diagnostic value of the ABCD, DiaRem, DiaBetter, Ad-DiaRem, and IMS scores and diabetes duration for partial diabetes remission in the SOS surgery group after 2 and 10 years of follow-up.
For partial diabetes remission at 10 years, the predictive ability of scores and diabetes duration alone was reduced, resulting in AUROCs of 0.70 (95% CI 0.64–0.77) for ABCD, 0.72 (0.65–0.78) for DiaRem, 0.71 (0.65–0.78) for Ad-DiaRem, 0.74 (0.68–0.80) for DiaBetter, 0.76 (0.70–0.81) for IMS, and 0.73 (0.67–0.78) for diabetes duration (Fig. 1, right). At this time point, the predictive capacity of diabetes duration alone was similar to all five scores (P for all comparisons not significant).
In a subgroup analysis, we assessed the predictive capacities of scores and diabetes duration alone for complete diabetes remission at 2 and 10 years (Supplementary Fig. 3). In this analysis, the IMS score had higher predictive capacity than diabetes duration at 2 years (P < 0.001), but the predictive capacity of diabetes duration alone was similar to all scores for prediction of 10-year diabetes remission (P for all comparisons not significant).
Performance of the scores in the SOS cohort were also examined by calibration; that is, the previously reported remission rates after 2 years for the DiaRem, Ad-DiaRem, and DiaBetter scores (18,21) were plotted against the observed 2-year remission rates in the SOS study (Supplementary Fig. 4). All three DiaRem-related scores overestimated diabetes remission compared with observed SOS rates (i.e., predicted remission rates were higher than observed SOS remission rates) (P < 0.001 for all scores). We were unable to include ABCD and IMS scores in the calibration analysis since 2-year remission rates have not previously been reported. Furthermore, duration could not be included since previous reports on remission rates were not detailed enough for the lower range of duration (21).
Preoperative Remission Scores and Diabetes Duration Alone as Predictors of Diabetes Complications
Register data on diabetes complications were available for all included participants, and Fig. 2 shows the predictive capacity of the scores and diabetes duration for development of diabetes complications over 15 years of follow-up. For development of microvascular diabetes complications, AUROCs were 0.70 (95% CI 0.62–0.78) for ABCD, 0.77 (0.70–0.84) for DiaRem, 0.78 (0.72–0.84) for Ad-DiaRem, 0.80 (0.73–0.86) for DiaBetter, 0.79 (0.73–0.85) for IMS, and 0.77 (0.71–0.83) for diabetes duration alone. The predictive capacities for development of macrovascular diabetes complications were markedly lower, with AUROCs of 0.62 (0.56–0.68) for ABCD, 0.69 (0.63–0.75) for DiaRem, 0.71 (0.65–0.76) for Ad-DiaRem, 0.66 (0.60–0.73) for DiaBetter, 0.67 (0.61–0.73) for IMS, and 0.66 (0.60–0.72) for diabetes duration alone. None of the scores displayed a higher predictive capacity than diabetes duration alone for microvascular complications (P for all comparisons not significant). Diabetes duration alone had marginally lower capacity than the Ad-DiaRem for prediction of macrovascular complications (P = 0.05) but similar capacity compared with the remaining four scores (P for all comparisons not significant).
Figure 2.
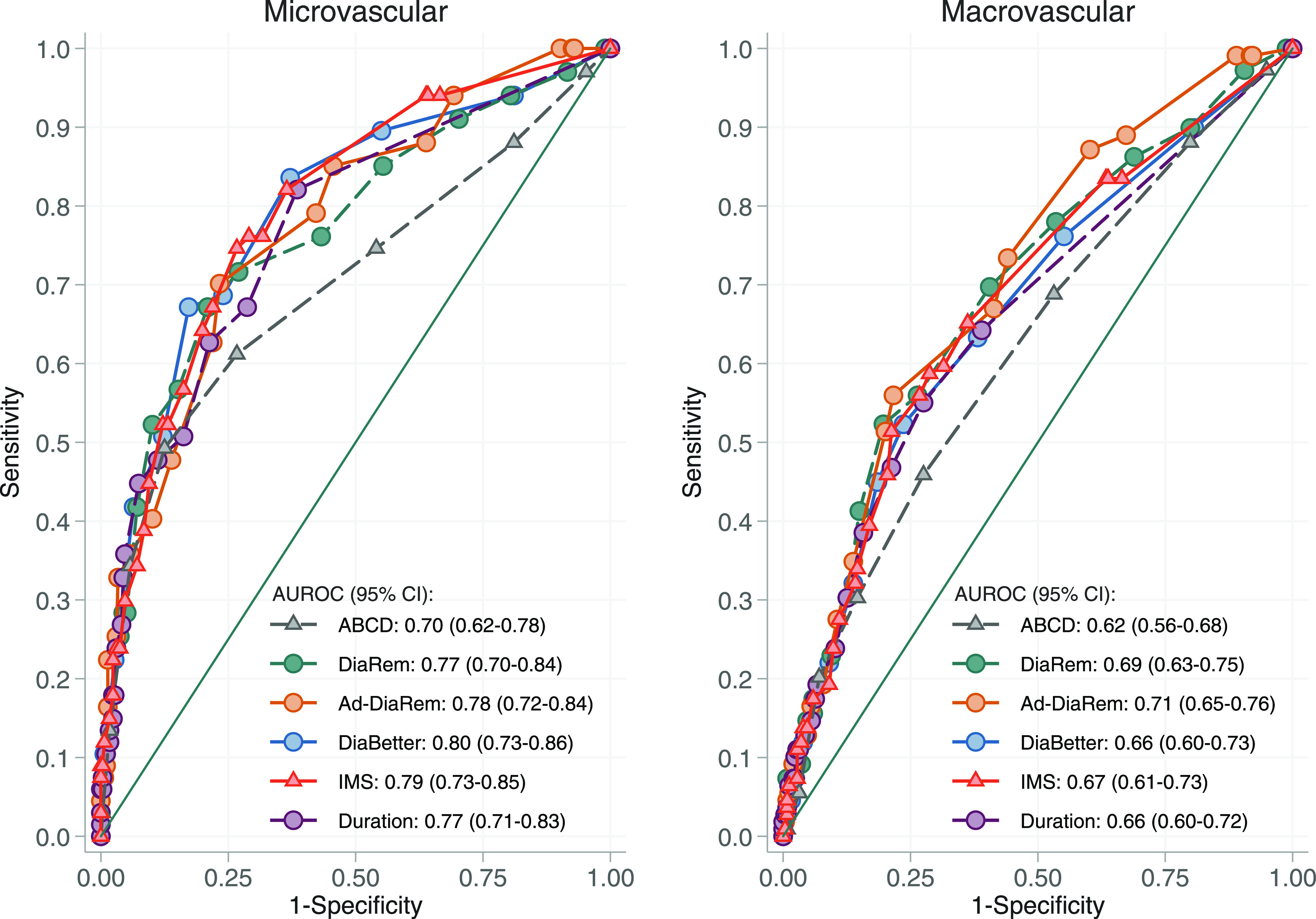
Comparison of the diagnostic value of the ABCD, DiaRem, DiaBetter, Ad-DiaRem, and IMS scores and diabetes duration for development of microvascular and macrovascular diabetes complications in the SOS surgery group over 15 years of follow-up.
Predictive Capacity in Relation to Type of Surgical Procedure
Scores included in this analysis were originally developed using GBP data, and we therefore specifically assessed the predictive capacities of scores and diabetes duration alone in the GBP and banding/VBG subgroups (Supplementary Table 3). It should be noted that numbers in the GBP subgroup are small and that the predictions should therefore be interpreted with caution. Only 39 individuals, 21 of whom were in remission, were available for assessment of remission at 10 years, and the analysis resulted in AUROCs between 0.72 and 0.77 for scores and an AUROC of 0.77 for diabetes duration alone. For assessment of diabetes complications over 15 years, 65 individuals in the GBP subgroup were available for assessment, and the analysis is based on only 8 microvascular and 18 macrovascular events. Prediction of microvascular diabetes complications resulted in AUROCs between 0.77 and 0.83 for scores and an AUROC of 0.80 for duration alone. Again, the predictive capacities for macrovascular complications were markedly lower (AUROCs between 0.56 and 0.64 for scores and AUROC of 0.63 for diabetes duration alone). Importantly, within each treatment subgroup, there were limited differences between prediction accuracy for scores and duration alone, regardless of studied outcome (Supplementary Table 3). Unfortunately, we were unable to test the capacity of scores and duration for prediction of remission at 2 years because of very few nonremission cases at this time point.
Discriminative Capacity and Estimated Optimal Score Cutoffs
Finally, we estimated optimal score cutoffs from SOS data using the Youden method for maximizing sensitivity and specificity. When assessing predictive ability of remission scores using SOS data, sensitivity/specificity >70% was achieved by all scores for 2-year remission and by the Ad-DiaRem and IMS scores for prediction of microvascular complications (Supplementary Table 4). However, none of the scores were able to reach a high level of discrimination regardless of analyzed outcome, and all five scores displayed poor predictive capacity for 10-year diabetes remission and macrovascular complications at optimal cutoffs. Youden estimates of diabetes duration cutoffs for various outcomes are included for comparison (Supplementary Table 4). However, given the high proportion of patients with screen-detected diabetes in the SOS study, these cutoffs should be interpreted with caution.
Conclusions
Our post hoc analysis of participants in the SOS study with preoperative type 2 diabetes who underwent bariatric surgery shows that the preoperative remission scores ABCD, DiaRem, Ad-DiaRem, DiaBetter, and IMS have good discriminatory power for prediction of short-term diabetes remission but not for prediction of diabetes remission at 10 years or risk of microvascular and macrovascular complications over 15 years of follow-up. In addition, the discriminatory capacity of diabetes duration alone was only slightly lower than the best performing score at the 2-year follow-up and similar to all five preoperative scores for prediction of 10-year diabetes remission and risk of microvascular diabetes complications over 15 years.
Our results confirm previous observations showing that remission scores have good discriminatory power in the short term (8,17,18,20,21,23,34) (Supplementary Table 5). For all scores except ABCD, we noted AUROCs of ≥0.85 at the 2-year follow-up, indicating excellent discrimination between those who will and will not achieve short-term remission. However, our calibration analysis comparing the reported 2-year remission rates as predicted by DiaRem, Ad-DiaRem, and DiaBetter with the observed remission rates in the SOS study showed that the previously predicted remission rates (18,21) were, in general, too optimistic. This is in line with a recent report that evaluated 11 different prediction models 1 year after sleeve gastrectomy and found that for the five scores included in the present report, remission rates were overestimated by 5–22% (34). Possible reasons for poor calibration include differences in case ascertainment and study population characteristics, indicating a need for recalibration of the scores before being more widely applied (31).
The ultimate treatment goal of diabetes treatment is not diabetes remission per se but to reduce the risk of serious diabetes complications. Thus, it is essential to include end-organ damage as an outcome when evaluating prediction models (1,35). To our knowledge, none of the scores assessed in the current report have previously been tested for prediction of diabetes complications. Here, we showed that the discriminatory capacity of remission scores and diabetes duration alone for development of microvascular and macrovascular diabetes complications over 15 years was limited. In combination with our results showing that none of the scores tested nor diabetes duration alone can be used to correctly identify those who will achieve durable remission, these results suggest that preoperative prediction models need to be improved before being used for overall prediction of long-term diabetes outcomes. The etiology of type 2 diabetes is complex, and factors such as genetic predisposition to reduced insulin secretion or impaired β-cell function, control systems for maintaining energy balance, or psychological or behavioral characteristics may need to be considered in future prediction models to improve clinical utility (36–38). It is possible that an extended approach that includes such factors could identify patients with a low chance of remission, enabling targeted intensified postoperative management to optimize postsurgery weight reduction and long-term glycemic control.
Optimal cutoffs for remission scores, indicating the highest possible prediction accuracy, could provide valuable tools for improvement of clinical guidelines. With respect to 2-year remission, optimal SOS cutoffs for the scores had acceptable discriminatory power, and Youden cutoffs were in the same range as those previously suggested for short-term (1–2-year) diabetes remission (17,20,34,39,40). Markedly lower levels of discrimination were found when using optimal SOS study cutoffs for prediction of 10-year remission for all scores, again indicating that preoperative prediction models need to be improved if the intention is to predict durable diabetes remission after bariatric surgery.
An optimal cutoff for diabetes duration would be an easy-to-use alternative to composite scores, but currently there is no clear recommendation. Diabetes duration cutoffs ranging between 2 and 10 years have previously been suggested to identify patients with a high chance of remission after bariatric surgery (9,11,13–15). Also, electronic health records on diabetes duration are not always available, and it could be argued that self-reported diabetes duration is not reliable and that incorporating that measure into prediction models may introduce errors. However, a recent study showed that there is a high agreement between self-reported and electronic health record–derived diabetes duration (8). In the SOS study, Youden estimates of optimal cutoffs for diabetes duration were as low as 1 or 2 years, depending on analyzed outcome, confirming the importance of early surgical intervention (3,25,26,41). However, it is important to note that a high proportion of patients in the SOS study had screen-detected diabetes and that the discriminatory power was low for prediction of both durable (10-year) remission and diabetes complications. These results should therefore be interpreted with caution and not viewed as clinically reliable duration cutoffs for treatment recommendations.
Important strengths of the SOS study are the very long follow-up of both diabetes status and diabetes complications and the prospective study design. Limitations include that the SOS study was initiated before repeated measurements were routinely used for the diagnosis of type 2 diabetes and that diagnosis is therefore based on a single time point. Also, there is a difference in mean preoperative diabetes duration in the SOS study (2.3 years) and in the retrospective cohorts used for Ad-DiaRem, DiaBetter, and IMS score generation (5–6 years) (17,18,20). Finally, it should be noted that the scoring models were developed using data from patients who underwent GBP, whereas the majority of SOS patients were treated with older surgical techniques (VBG or banding). Given that the average weight reduction and short-term remission rate after VBG/banding, as well as after sleeve gastrectomy, are lower than after GBP, predictive capacities of scores for diabetes remission will likely be higher in a GBP cohort (14,42,43). This is in line with our findings for some of the models, although these results should be interpreted with caution because of the small number of patients treated with GBP in the SOS study. On the other hand, it is inevitable that surgical techniques are updated and refined over time, meaning that interpretation of results from long-term studies, such as the one presented here, will always be affected by procedural changes. Reports have suggested that both the DiaRem and the Ad-DiaRem scores are useful tools for short-term prediction of diabetes remission in patients treated with sleeve gastrectomy or gastric banding (20,21) and that remaining differences in discriminatory capacity may partly be due to more limited weight reduction in patients treated with banding (21). Nevertheless, future studies in larger cohorts treated with current surgical techniques and with a high variability of diabetes duration and long follow-up will be needed to assess generalizability of our results.
In conclusion, our results from the SOS study suggest that the remission scores ABCD, DiaRem, Ad-DiaRem, DiaBetter, and IMS and diabetes duration alone are good predictors of short-term diabetes remission. However, both remission scores and diabetes duration alone have limited predictive ability to correctly identify those who will achieve durable remission and have lower risk for diabetes-related complications.
Article Information
Acknowledgments. The authors thank the staff members at 480 primary health care centers and 25 surgical departments in Sweden who participated in the SOS study. The authors acknowledge Christina Torefalk and Björn Henning for administrative support and Rosie Perkins (all from Institute of Medicine, University of Gothenburg, Gothenburg, Sweden) for editing the report.
Funding. Research reported in this publication was supported by the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-717881 and ALFGBG-717891), the Swedish Research Council (2017-01707), the Swedish Diabetes Foundation (2019-417), and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (award number R01-DK-105948). C.W.l.R. received funding from the ALF agreement, Swedish Research Council, Science Foundation Ireland, Health Research Board, and Irish Research Council.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. L.M.S.C. has received consulting fees from AstraZeneca and Johnson & Johnson. C.W.l.R. served on advisory boards for Novo Nordisk, GI Dynamics, Keyron, Sanofi, and Consilient Health. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.S. wrote the manuscript with contributions from all authors. K.S., L.M.S.C., and M.P. designed the study. K.S., M.T., and C.W.l.R. were responsible for HbA1c analyses. M.P. designed and conducted the statistical analyses. All authors were involved in acquisition and interpretation of data, critically reviewed the report, contributed to the revision, and approved the final version. K.S. and M.P. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this article were presented at ObesityWeek, Nashville, TN, 11–15 November 2018, and the European Congress on Obesity, Glasgow, U.K., 28 April–1 May 2019.
Footnotes
Clinical trial reg. no. NCT01479452, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.12765875.
References
- 1.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med 1993;328:1676–1685 [DOI] [PubMed] [Google Scholar]
- 2.Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol 2018;71:670–687 [DOI] [PubMed] [Google Scholar]
- 3.Sjöström L, Peltonen M, Jacobson P, et al. . Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304 [DOI] [PubMed] [Google Scholar]
- 4.Dixon JB, Chuang LM, Chong K, et al. . Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care 2013;36:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aminian A, Brethauer SA, Andalib A, et al. . Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg 2017;266:650–657 [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Hur KY, Lakadawala M, et al. . Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis 2013;9:379–384 [DOI] [PubMed] [Google Scholar]
- 7.Panunzi S, Carlsson L, De Gaetano A, et al. . Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care 2016;39:166–174 [DOI] [PubMed] [Google Scholar]
- 8.Still CD, Benotti P, Mirshahi T, Cook A, Wood GC. DiaRem2: incorporating duration of diabetes to improve prediction of diabetes remission after metabolic surgery. Surg Obes Relat Dis 2019;15:717–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nautiyal HK, Guan W, Lin S, Liang H. Preoperative predictors of early relapse/no-remission of type-2 diabetes after metabolic surgery in Chinese patients. Clin Obes 2020;10:e12350. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez A, Casamitjana R, Flores L, et al. . Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg 2012;256:1023–1029 [DOI] [PubMed] [Google Scholar]
- 11.Umemura A, Sasaki A, Nitta H, et al. . Prognostic factors and a new preliminary scoring system for remission of type 2 diabetes mellitus after laparoscopic sleeve gastrectomy. Surg Today. 13 March 2020 [Epub ahead of print]. DOI: 10.1007/s00595-020-01990-z [DOI] [PubMed] [Google Scholar]
- 12.Dang JT, Sheppard C, Kim D, et al. . Predictive factors for diabetes remission after bariatric surgery. Can J Surg 2019;62:315–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminian A, Brethauer SA, Kashyap SR, Kirwan JP, Schauer PR. DiaRem score: external validation. Lancet Diabetes Endocrinol 2014;2:12–13 [DOI] [PubMed] [Google Scholar]
- 14.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegenga H, Haines A, Jones K, Wilding J; Guideline Development Group . Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ 2014;349:g6608. [DOI] [PubMed] [Google Scholar]
- 16.Still CD, Wood GC, Benotti P, et al. . Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2014;2:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aron-Wisnewsky J, Sokolovska N, Liu Y, et al. . The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia 2017;60:1892–1902 [DOI] [PubMed] [Google Scholar]
- 18.Pucci A, Tymoszuk U, Cheung WH, et al. . Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: the role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet Med 2018;35:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood GC, Mirshahi T, Still CD, Hirsch AG. Association of DiaRem score with cure of type 2 diabetes following bariatric surgery. JAMA Surg 2016;151:779–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig Wood G, Horwitz D, Still CD, et al. . Performance of the DiaRem score for predicting diabetes remission in two health systems following bariatric surgery procedures in Hispanic and non-Hispanic white patients. Obes Surg 2018;28:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dicker D, Golan R, Aron-Wisnewsky J, et al. . Prediction of long-term diabetes remission after RYGB, sleeve gastrectomy, and adjustable gastric banding using DiaRem and advanced-DiaRem scores. Obes Surg 2019;29:796–804 [DOI] [PubMed] [Google Scholar]
- 22.Kam H, Tu Y, Pan J, et al. . Comparison of four risk prediction models for diabetes remission after Roux-en-Y gastric bypass surgery in obese Chinese patients with type 2 diabetes mellitus. Obes Surg 2020;30:2147–2157 [DOI] [PubMed] [Google Scholar]
- 23.Debédat J, Sokolovska N, Coupaye M, et al. . Long-term relapse of type 2 diabetes after roux-en-Y gastric bypass: prediction and clinical relevance. Diabetes Care 2018;41:2086–2095 [DOI] [PubMed] [Google Scholar]
- 24.Chen JC, Hsu NY, Lee WJ, Chen SC, Ser KH, Lee YC. Prediction of type 2 diabetes remission after metabolic surgery: a comparison of the individualized metabolic surgery score and the ABCD score. Surg Obes Relat Dis 2018;14:640–645 [DOI] [PubMed] [Google Scholar]
- 25.Carlsson LM, Peltonen M, Ahlin S, et al. . Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704 [DOI] [PubMed] [Google Scholar]
- 26.Carlsson LMS, Sjöholm K, Karlsson C, et al. . Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol 2017;5:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buse JB, Caprio S, Cefalu WT, et al. . How do we define cure of diabetes? Diabetes Care 2009;32:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludvigsson JF, Andersson E, Ekbom A, et al. . External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merlo J, Lindblad U, Pessah-Rasmussen H, et al. . Comparison of different procedures to identify probable cases of myocardial infarction and stroke in two Swedish prospective cohort studies using local and national routine registers. Eur J Epidemiol 2000;16:235–243 [DOI] [PubMed] [Google Scholar]
- 30.Neovius M, Simard J, Sundström A, et al.; ARTIS Study Group . Generalisability of clinical registers used for drug safety and comparative effectiveness research: coverage of the Swedish Biologics Register. Ann Rheum Dis 2011;70:516–519 [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Borisenko O, Telegina I, et al. . Systematic review of risk prediction models for diabetes after bariatric surgery. Br J Surg 2016;103:1420–1427 [DOI] [PubMed] [Google Scholar]
- 32.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35 [DOI] [PubMed] [Google Scholar]
- 33.Lee WJ, Chong K, Chen SC, et al. . Preoperative prediction of type 2 diabetes remission after gastric bypass surgery: a comparison of DiaRem scores and ABCD scores. Obes Surg 2016;26:2418–2424 [DOI] [PubMed] [Google Scholar]
- 34.Shen SC, Wang W, Tam KW, et al. . Validating risk prediction models of diabetes remission after sleeve gastrectomy. Obes Surg 2019;29:221–229 [DOI] [PubMed] [Google Scholar]
- 35.Coleman KJ, Haneuse S, Johnson E, et al. . Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care 2016;39:1400–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirdah MM, Reading NS. Genetic predisposition in type 2 diabetes: a promising approach toward a personalized management of diabetes. Clin Genet. 8 May 2020 [Epub ahead of print]. DOI: 10.1111/cge.13772 [DOI] [PubMed] [Google Scholar]
- 37.David LA, Sijercic I, Cassin SE. Preoperative and post-operative psychosocial interventions for bariatric surgery patients: a systematic review. Obes Rev 2020;21:e12926. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen KT, Korner J. The sum of many parts: potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep 2014;14:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honarmand K, Chetty K, Vanniyasingam T, Anvari M, Chetty VT. Type 2 diabetes remission rates 1-year post-Roux-en-Y gastric bypass and validation of the DiaRem score: the Ontario Bariatric Network experience. Clin Obes 2017;7:176–182 [DOI] [PubMed] [Google Scholar]
- 40.Ahuja A, Tantia O, Chaudhuri T, et al. . Predicting remission of diabetes post metabolic surgery: a comparison of ABCD, DiaRem, and DRS scores. Obes Surg 2018;28:2025–2031 [DOI] [PubMed] [Google Scholar]
- 41.Jans A, Näslund I, Ottosson J, Szabo E, Näslund E, Stenberg E. Duration of type 2 diabetes and remission rates after bariatric surgery in Sweden 2007-2015: a registry-based cohort study. PLoS Med 2019;16:e1002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchwald H, Estok R, Fahrbach K, et al. . Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 [DOI] [PubMed] [Google Scholar]
- 43.Arterburn D, Wellman R, Emiliano A, et al.; PCORnet Bariatric Study Collaborative . Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med 2018;169:741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]


