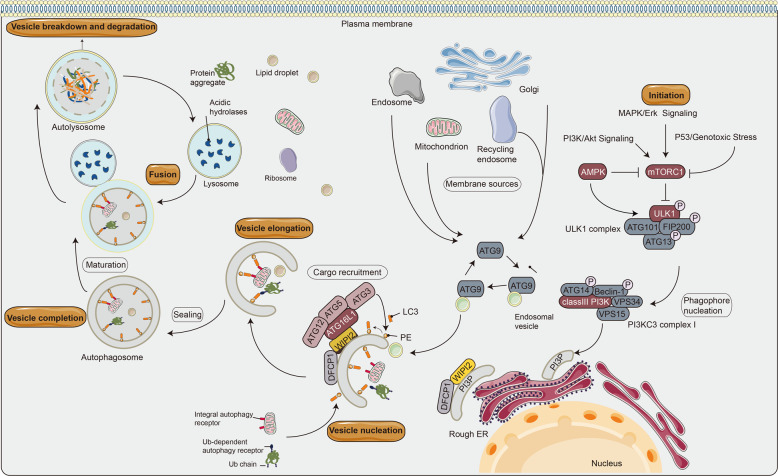
Fig. 1. Schematic diagram of the autophagy process in mammalian cells.
The mTOR complex 1 (mTORC1) contributes to the initiation of autophagy, integrates upstream signals such as PI3K/Akt pathway, AMPK, P53, and Bcl-2 protein family, which play different regulatory roles in autophagy135. The ULK1 complex induces vesicle nucleation and translocates to a characteristic endoplasmic reticulum (ER) structure called omegasome, where it phosphorylates PI3KC3 complex I to produce phosphatidylinositol-3-phosphate (PI3P) in omegasome. Specifically, Beclin1, a Bcl-2-homology (BH)-3 domain-only protein, is phosphorylated by ULK1 and acts as a scaffold for the PI3KC3 complex I, which facilitates localization of autophagy proteins to the phagophore. Atg9 is a transmembrane protein, which participates in the early stage of phagophore formation. PI3P recruits specific autophagy effectors, such as WIPIs (mammalian homolog of yeast Atg18) and zinc finger FYVE-type containing 1 (DFCP1). WIPIs directly binds to ATG16L1 under the regulation of the ubiquitin-like conjugation system to form the ATG12-ATG5-ATG16L1 complex and LC3 (mammalian homolog of yeast Atg8)-phosphatidylethanolamine (PE) binding. Ultimately, the isolation membrane is elongated and closed to form the autophagosome136. This binding reaction results in the conversion of LC3-I to LC3-II, a common autophagosome marker. When the autophagosome matures, it sheds the ATG proteins and fuses with the lysosome to produce autophagolysosome. Both the inner membrane of the autophagic vesicle and the luminal contents are degraded by lysosomal hydrolases (cathepsins B, D, and L). The resulting monomer molecules (such as amino acids and lipids) are recycled into the cytoplasm for reuse137. The pointed and blunt arrowheads indicate activation and inhibitory interactions, respectively. Ub, ubiquitin.