Abstract
Background
High-sensitivity C-reactive protein (hs-CRP) elevation frequently occurs in acute myocardial infarction (AMI) and is associated with adverse outcomes. Since diabetes mellitus (DM) is characterized by an underlying chronic inflammation, hs-CRP may have a different prognostic power in AMI patients with and without DM.
Methods
We prospectively included 2064 AMI patients; hs-CRP was measured at hospital admission. Patients were grouped according to hs-CRP quartiles and DM status. The primary endpoint was a composite of in-hospital mortality, cardiogenic shock, and acute pulmonary edema. Two-year all-cause mortality was the secondary endpoint.
Results
Twenty-six percent (n = 548) of patients had DM and they had higher hs-CRP levels than non-DM patients (5.32 vs. 3.24 mg/L; P < 0.0001). The primary endpoint incidence in the overall population (7%, 9%, 13%, 22%; P for trend < 0.0001), in DM (14%, 9%, 21%, 27%; P = 0.0001), and non-DM (5%, 8%, 10%, 19%; P < 0.0001) patients increased in parallel with hs-CRP quartiles. The adjusted risk of the primary endpoint increased in parallel with hs-CRP quartiles in DM and non-DM patients but this relationship was less evident in DM patients. In the overall population, the adjusted OR of the primary endpoint associated with an hs-CRP value ≥ 2 mg/L was 2.10 (95% CI 1.46-3.00). For the same risk, hs-CRP was 7 and 2 mg/L in patients with and without DM. A similar behavior was observed for the secondary endpoint when the HR associated with an hs-CRP value ≥ 2 mg/L found in the overall population was 2.25 (95% CI 1.57-3.22). For the same risk, hs-CRP was 8 and 1.5 mg/L in DM and non-DM patients.
Conclusions
This study shows that hs-CRP predicts in-hospital outcome and two-year mortality in AMI patients with and without DM. However, in DM patients, the same risk of developing events as in non-DM patients is associated to higher hs-CRP levels.
Keywords: Acute myocardial infarction, Inflammation, High-sensitivity C-reactive protein, Diabetes mellitus
Introduction
Type 2 diabetes mellitus (DM) is a common comorbidity in acute myocardial infarction (AMI), and it is associated with two-fold higher in-hospital and long-term mortality rates and with a higher risk of recurrent cardiovascular events [1–3]. Both DM and atherosclerosis are multifactorial conditions, which share a common inflammatory basis [4]. Indeed, on the one hand, DM is an independent risk factor for AMI and is considered a state of low-grade inflammation [5, 6]. On the other hand, inflammation plays a critical role in all phases of coronary athero-thrombosis, including plaque progression, rupture, and thrombosis leading to AMI [7].
C-reactive protein (CRP), an acute phase protein secreted by the liver, is the most widely used biomarker for detecting inflammatory conditions [8]. The elevation of CRP levels frequently occurs in AMI, and it has been associated with adverse outcomes, including higher risk of major adverse cardiac events, cardiovascular death, chronic kidney disease progression, acute kidney injury, and all-cause mortality [9–13]. To assess cardiovascular risk, physicians have now adopted high-sensitivity CRP (hs-CRP), instead of standard CRP assays that monitor infections and other inflammatory conditions [9]. In particular, in the AMI setting, hs-CRP demonstrated to be a more reliable indicator of outcome than CRP measured through traditional assays [11–15], showing that even a mild increase (≥ 2 mg/L) is of prognostic relevance [16].
Elevated hs-CRP levels in AMI patients may reflect a variable combination of chronic and acute (due to the ongoing cardiac event) inflammation. Since DM is more frequently associated with some degree of chronic inflammation, it is possible that, in AMI patients with DM, hs-CRP has a different prognostic relevance as compared to their non-DM counterpart.
The purpose of this study was to investigate the association between hs-CRP levels, measured at hospital admission, and in-hospital outcome and two-year mortality in a cohort of AMI patients according to DM status.
Materials and methods
Study population
This was a prospective, observational study. We enrolled all consecutive patients with AMI (n = 2178), both ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI), admitted to the Intensive Cardiac Care Unit of Centro Cardiologico Monzino in Milan between June 1, 2012 and October 1, 2017. Patients experiencing AMI as a complication of elective percutaneous coronary intervention (PCI) (Type 4a AMI) and those with concomitant systemic inflammatory conditions, including active infections (n = 88) or malignancies (n = 26) were excluded. The study complied with the Declaration of Helsinki, and the Ethics Committee of our center approved the research protocol (n. R520-CCM549). Written informed consent was obtained from all participants.
Study protocol
Patients were considered as suffering from DM if one of the following conditions were present: personal history of DM reported in clinical record, treatment with glucose lowering drugs, or a glycated hemoglobin value ≥ 6.5% (48 mmol/mol). Glycated hemoglobin was measured at hospital admission in all patients as a part of our routine laboratory package using a method NGSP certified and standardized to the DCCT assay [17].
High-sensitivity-CRP was measured at hospital admission by Cobas® assay (particle-enhanced immunoturbidimetric assay) on Cobas c501 (Roche) [18]. A hs-CRP value ≥ 2 mg/L was considered a sign of inflammation [16].
Study patients received medical treatment and coronary revascularization based on the current standards of care recommended by published guidelines on AMI [19]. Demographical, clinical, biochemical data, and echocardiographic left ventricular ejection fraction (LVEF) were collected at hospital admission. After hospital discharge, all patients were followed-up for 2 years. Patient follow-up was mainly obtained through regularly scheduled outpatient visits or, in a minority of cases, by telephone calls performed by dedicated medical personnel.
The primary endpoint of the study was a composite of in-hospital mortality, cardiogenic shock, and acute pulmonary edema. Cardiogenic shock was defined as persistent systolic arterial pressure ≤ 80 mmHg and evidence of vital organ hypoperfusion caused by severe left ventricular dysfunction, right ventricular infarction, or mechanical complications of infarction, and not due to hypovolemia, hemorrhage, bradyarrhythmias, or tachyarrhythmias. Acute pulmonary edema was defined as respiratory distress, tachypnea, and orthopnea with rales over the lung fields and arterial oxygen saturation < 90%. To avoid interference, each patient could only account for one event classification. Two-year all-cause mortality was the secondary endpoint of the study.
Statistical analysis
A sample size of 2000 patients was calculated under the following assumptions: 10% overall incidence of the primary endpoint [1–3], and an expected odds ratio (OR) increasing by a 1.5 factor from the first to the fourth hs-CRP quartile in the overall population. This sample size allowed an 85% statistical power in assessing a significant difference (α error of 0.05) of the primary endpoint between the two quartiles. Moreover, this sample size allowed a 90% statistical power when an overall incidence of 20% of two-year all-cause mortality was considered [20],with an expected 20% higher mortality risk (hazard ratio [HR] 1.2) between the first and the fourth hs-CRP quartile.
Continuous variables are presented as mean ± SD. Variables with a skewed distribution are presented as median and interquartile ranges. Categorical data are presented as n (%). Trends across hs-CRP quartiles were assessed by ANCOVA and by Mantel–Haenszel Chi square, as appropriate. The association between hs-CRP and study endpoints was assessed by logistic regression analysis. Results are presented as OR with 95% confidence intervals (CI). Cox proportional hazard model was also used to assess HR and 95% CI for two-year mortality associated with hs-CRP quartiles. We calculated the P value for interaction between DM status and hs-CRP quartiles by logistic regression analysis and by Cox proportional hazard model, as appropriate. Pearson coefficient was used to assess the correlation between continuous variables. All analyses were performed in the overall study population as well as in DM and non-DM patients considered separately.
Kaplan–Meier analysis was used to generate time-to-event curves for two-year mortality in patients with hs-CRP < 2 mg/L or ≥ 2 mg/L. Log rank test was used to compare strata.
All analyses were adjusted according to an epidemiological model including the variables most closely associated with prognosis in AMI patients with and without DM [3]: LVEF ≤ 40%, estimated glomerular filtration rate (MDRD equation, based on age, gender, and serum creatinine concentration) ≤ 60 ml/min/1.73 m2, and AMI type (STEMI vs. NSTEMI). Moreover, we included in the model previous statin therapy due to its well-known anti-inflammatory effects [21].
Receiver-operating characteristics (ROC) curves were constructed to assess the sensitivity and specificity throughout the concentrations of hs-CRP to predict both primary and secondary endpoints.
Cochran-Mantel–Haenszel estimator was implemented to calculate the adjusted relative risk (RR) of two-year mortality in patients with and without DM.
A bootstrap approach with 2000 resamples was implemented to assess that, in classifying primary and secondary endpoints, the estimated best cutoff of hs-CRP values was consistently higher in DM patients than in non-DM patients.
All tests were 2-tailed, and a P < 0.05 was required for statistical significance. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Two-thousand-sixty-four AMI patients (mean age 67 ± 12 years, 1516 men, 1016 STEMI) were enrolled in the study. Of them, 548 (26%) had DM and 1366 (66%) had hs-CRP levels ≥ 2 mg/L. Inflammation (hs-CRP ≥ 2 mg/L) was more frequent in DM patients than in non-DM patients (74% vs. 64%; P < 0.0001). The baseline clinical characteristics and in-hospital outcomes of patients with and without DM and of those with hs-CRP ≥ and < 2 mg/L are shown in Tables 1 and 2, respectively. Patients with DM were older, more likely to have comorbidities, prior cardiovascular events and higher admission hs-CRP levels than those without DM, despite an almost two-fold higher rate of chronic statin therapy. Moreover, DM patients had a more complicated in-hospital clinical course. Similar differences in clinical characteristics and in-hospital outcomes were observed in patients with hs-CRP ≥ 2 mg/L when compared to those with hs-CRP < 2 mg/L. High-sensitivity-troponin I (hs-TnI) peak value was similar in DM and non-DM patients (43,153 ± 82,894 and 45,392 ± 99,242 ng/L, respectively; P = 0.64) and in patients with and without inflammation (46,427 ± 98,296 and 41,582 ± 89,099 ng/L, respectively; P = 0.28). In the entire population, a significant correlation between admission hs-CRP and hs-TnI peak value was found (r = 0.11; P < 0.0001). This relationship was stronger in non-DM patients (r = 0.12; P < 0.0001) than in DM patients (r = 0.07; P = 0.08).
Table 1.
Baseline clinical characteristics and in-hospital outcomes of the study patients according to the presence of diabetes mellitus
| DM (n = 548) | Non-DM (n = 1516) | P value | |
|---|---|---|---|
| Age (years) | 70 ± 11 | 66 ± 13 | < 0.0001 |
| Male sex, n (%) | 430 (78%) | 1086 (72%) | 0.002 |
| Body mass index (kg/m2) | 28 ± 5 | 26 ± 4 | < 0.0001 |
| Hypertension, n (%) | 439 (80%) | 897 (59%) | < 0.0001 |
| Smokers, n (%) | 166 (30%) | 524 (35%) | 0.07 |
| Dyslipidemia, n (%) | 348 (64%) | 682 (45%) | < 0.0001 |
| STEMI, n (%) | 237 (43%) | 779 (51%) | 0.001 |
| Prior MI, n, (%) | 204 (37%) | 331 (22%) | < 0.0001 |
| Prior CABG, n (%) | 110 (20%) | 141 (9%) | < 0.0001 |
| Prior PCI, n (%) | 199 (36%) | 319 (21%) | < 0.0001 |
| LVEF (%) | 48 ± 12 | 51 ± 12 | < 0.0001 |
| Time-to-presentation (hours) | 12.9 ± 26.5 | 12.3 ± 26.4 | 0.64 |
| CA/PCI in hospital, n (%) | 495 (90%) | 1436 (95%) | 0.0005 |
| Laboratory values at hospital admission | |||
| hs-CRP (mg/L) | 5.32 (1.86–21.51) | 3.24 (1.35–10.03) | < 0.0001 |
| Blood glucose (mg/dl) | 202 ± 82 | 133 ± 42 | < 0.0001 |
| HbA1c (%) | 7.4 ± 1.7 | 5.5 ± 0.4 | < 0.0001 |
| Serum creatinine (mg/dl) | 1.02 (0.8–1.3) | 0.92 (0.8–1.1) | < 0.0001 |
| eGFR (ml/min/1.73 m2) | 73 ± 32 | 80 ± 25 | < 0.0001 |
| Hemoglobin (g/dl) | 13 ± 2 | 14 ± 2 | < 0.0001 |
| hs-Tn I (ng/L) | 6824 ± 34,686 | 5875 ± 21,937 | 0.48 |
| Medication before AMI | |||
| Statins, n (%) | 275 (51%) | 415 (28%) | < 0.0001 |
| ACEi/ARB, n (%) | 271 (49%) | 541 (36%) | < 0.0001 |
| Beta-blockers, n (%) | 281 (51%) | 452(30%) | < 0.0001 |
| Aspirin, n (%) | 307 (56%) | 443 (29%) | < 0.0001 |
| In-hospital complications | |||
| Death, n (%) | 14 (2.6%) | 26 (1.7%) | 0.22 |
| Cardiogenic shock, n (%) | 41 (7%) | 76 (5%) | 0.03 |
| Acute pulmonary edema, n (%) | 92 (17%) | 122 (8%) | < 0.0001 |
| Mechanical ventilation, n (%) | 28 (5%) | 50 (3%) | 0.06 |
| Atrial fibrillation, n (%) | 79 (14%) | 130 (9%) | 0.0005 |
| VT/VF, n (%) | 28 (5%) | 140 (9%) | 0.002 |
| High-degree AV block, n (%) | 25 (5%) | 49 (3%) | 0.15 |
| Major bleeding, n (%) | 36 (7%) | 36 (2%) | < 0.0001 |
| Medication at hospital discharge | |||
| Dual antiplatelet therapy, n (%) | 488 (91%) | 1444 (97%) | < 0.0001 |
| Statins, n (%) | 491 (92%) | 1363 (91%) | 0.74 |
| Beta-blockers, n (%) | 444 (83%) | 1138 (77%) | 0.002 |
| ACEi/ARB, n (%) | 356 (67%) | 901 (61%) | 0.01 |
ACEi Angiotensin-converting enzyme inhibitors, ARB Angiotensin II receptor blockers, AV Atrio-ventricular, CA Coronary angiography, CABG Coronary artery bypass graft, DM Diabetes mellitus, eGFR Estimated glomerular filtration rate, HbA1c Glycated haemoglobin, hs-CRP High-sensitivity C-reactive protein, hs-TnI High-sensitivity troponin I, LVEF Left ventricular ejection fraction, MI Myocardial infarction, PCI Percutaneous coronary intervention, STEMI ST-segment elevation myocardial infarction, VT/VF Ventricular tachycardia/ventricular fibrillation
Table 2.
Baseline clinical characteristics and in-hospital outcomes of the study patients according to hs-CRP value at hospital admission
| Hs-CRP > 2 mg/L (n = 1366) | Hs-CRP < 2 mg/L (n = 698) | P value | |
|---|---|---|---|
| Age (years) | 68 ± 12 | 65 ± 12 | < 0.0001 |
| Male sex, n (%) | 980 (72%) | 536 (77%) | 0.01 |
| Body mass index (kg/m2) | 27 ± 5 | 26 ± 4 | < 0.0001 |
| Hypertension, n (%) | 920 (67%) | 416 (60%) | 0.0004 |
| Diabetes mellitus, n (%) | 403 (29%) | 145 (21%) | < 0.0001 |
| Smokers, n (%) | 474 (35%) | 216 (31%) | 0.0001 |
| Dyslipidemia, n (%) | 680 (50%) | 350 (50%) | 0.90 |
| STEMI, n (%) | 683 (50%) | 333 (48%) | 0.32 |
| Prior MI, n, (%) | 337 (25%) | 198 (28%) | 0.07 |
| Prior CABG, n (%) | 175 (13%) | 76 (11%) | 0.20 |
| Prior PCI, n (%) | 308 (23%) | 210 (30%) | 0.0002 |
| LVEF (%) | 49 ± 12 | 52 ± 11 | < 0.0001 |
| Time-to-presentation (hours) | 12.9 ± 25.1 | 12.2 ± 27.5 | 0.56 |
| CA/PCI in hospital, n (%) | 1260 (92%) | 671 (96%) | 0.0008 |
| Laboratory values at hospital admission | |||
| hs-CRP (mg/L) | 7.37 (3.80–24.11) | 1.03 (0.67–1.45)- | |
| Blood glucose (mg/dl) | 157 ± 68 | 141 ± 52 | < 0.0001 |
| HbA1c (%) | 6.1 ± 1.3 | 5.9 ± 1.1 | 0.001 |
| Serum creatinine (mg/dl) | 0.95 (0.8–1.2) | 0.92 (0.8–1.1) | 0.001 |
| eGFR (ml/min/1.73 m2) | 76 ± 27 | 82 ± 26 | < 0.0001 |
| Hemoglobin (g/dl) | 13 ± 2 | 14 ± 2 | < 0.0001 |
| hs-Tn I (ng/L) | 7485 ± 27,414 | 3468 ± 21,357 | 0.001 |
| Medication before AMI | |||
| Statins, n (%) | 429 (32%) | 262 (38%) | 0.001 |
| ACEi/ARB, n (%) | 528 (39%) | 284 (41%) | 0.36 |
| Beta-blockers, n (%) | 498 (36%) | 235 (34%) | 0.21 |
| Aspirin, n (%) | 496 (36%) | 254 (36%) | 0.96 |
| In-hospital complications | |||
| Death, n (%) | 30 (2.2%) | 10 (1.4%) | 0.23 |
| Cardiogenic shock, n (%) | 90 (7%) | 27 (4%) | 0.01 |
| Acute pulmonary edema, n (%) | 179 (13%) | 35 (5%) | < 0.0001 |
| Mechanical ventilation, n (%) | 58 (4%) | 20 (3%) | 0.12 |
| Atrial fibrillation, n (%) | 160 (12%) | 49 (7%) | < 0.0001 |
| VT/VF, n (%) | 101 (7%) | 67 (10%) | 0.08 |
| High-degree AV block, n (%) | 54 (4%) | 20 (3%) | 0.21 |
| Major bleeding, n (%) | 59 (4%) | 13 (2%) | 0.004 |
| Medication at hospital discharge | |||
| Dual antiplatelet therapy, n (%) | 1201 (90%) | 623 (91%) | 0.64 |
| Statins, n (%) | 1214 (91%) | 640 (92%) | 0.30 |
| Beta-blockers, n (%) | 444 (83%) | 1138 (77%) | 0.002 |
| ACEi/ARB, n (%) | 839 (63%) | 418 (60%) | 0.27 |
ACEi Angiotensin-converting enzyme inhibitors, ARB Angiotensin II receptor blockers, AV Atrio-ventricular, CA Coronary angiography, CABG Coronary artery bypass graft, eGFR Estimated glomerular filtration rate, HbA1c Glycated haemoglobin, hs-CRP High-sensitivity C-reactive protein, hs-TnI high-sensitivity troponin I, LVEF left ventricular ejection fraction, MI myocardial infarction, PCI percutaneous coronary intervention, STEMI ST-segment elevation myocardial infarction, VT/VF ventricular tachycardia /ventricular fibrillation
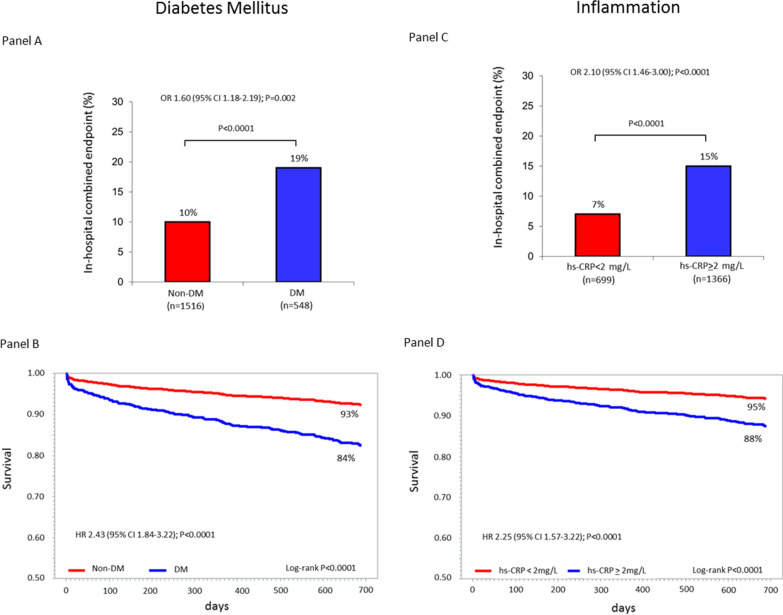
The incidence of the primary and secondary endpoints in patients with and without DM and in those with hs-CRP ≥ and < 2 mg/L is reported in Fig. 1. Both DM and inflammation had a significantly higher adjusted risk of the two study endpoints.
Fig. 1.
Panel A: incidence of the in-hospital combined clinical endpoint (death, cardiogenic shock, and acute pulmonary edema) in patients with and without diabetes mellitus (DM) and adjusted odds ratio (OR) and 95% confidence interval (CI) associated with DM. Panel B: Kaplan–Meier survival curves stratified by DM status and adjusted hazard ratio (HR) and 95% CI associated with DM. Panel C: incidence of the in-hospital combined clinical endpoint (death, cardiogenic shock, and acute pulmonary edema) in patients with high-sensitivity C-reactive protein (hs-CRP) ≥ and < 2 mg/L and adjusted OR and 95% CI associated with a hs-CRP value ≥ 2 mg/L. Panel D: Kaplan–Meier survival curves stratified by hs-CRP cut-off value (2 mg/L) and adjusted HR and 95% CI associated with a hs-CRP value ≥ 2 mg/L. All analyses were adjusted for left ventricular ejection fraction (≤ or > 40%), estimated glomerular filtration rate (≤ or > 60 ml/min/1.73 m2), type of acute myocardial infarction (STEMI vs. NSTEMI) and prior statin use
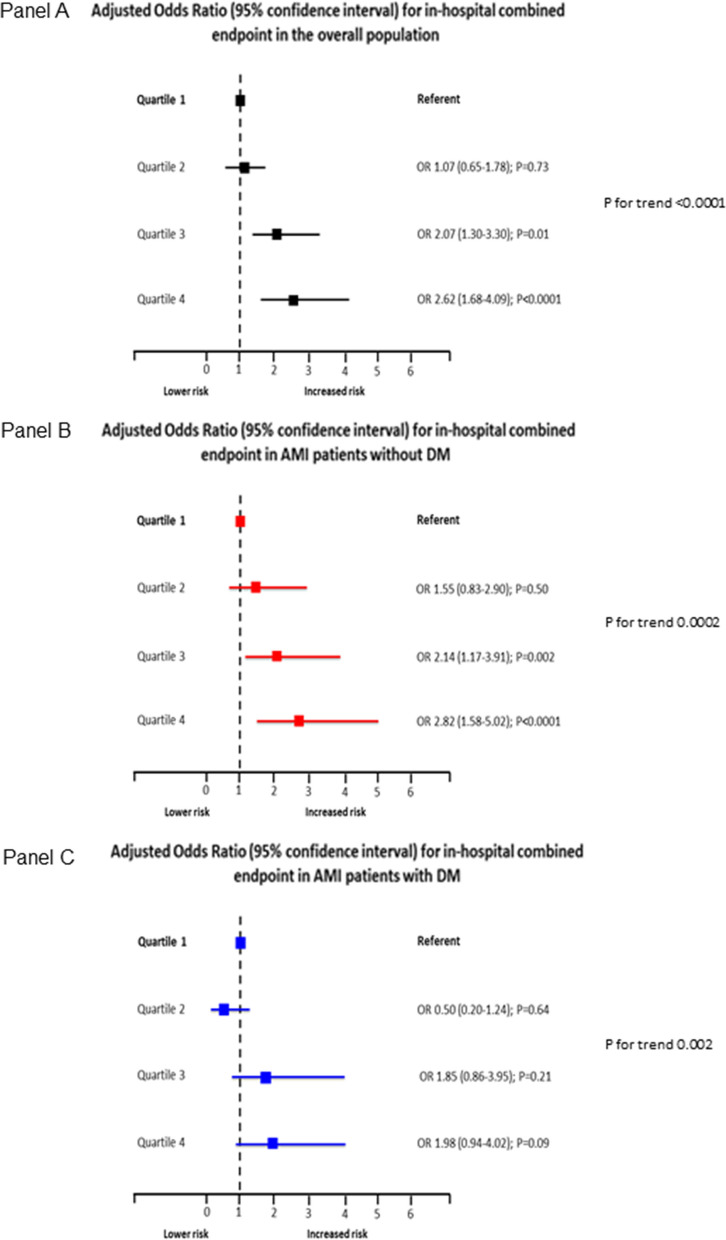
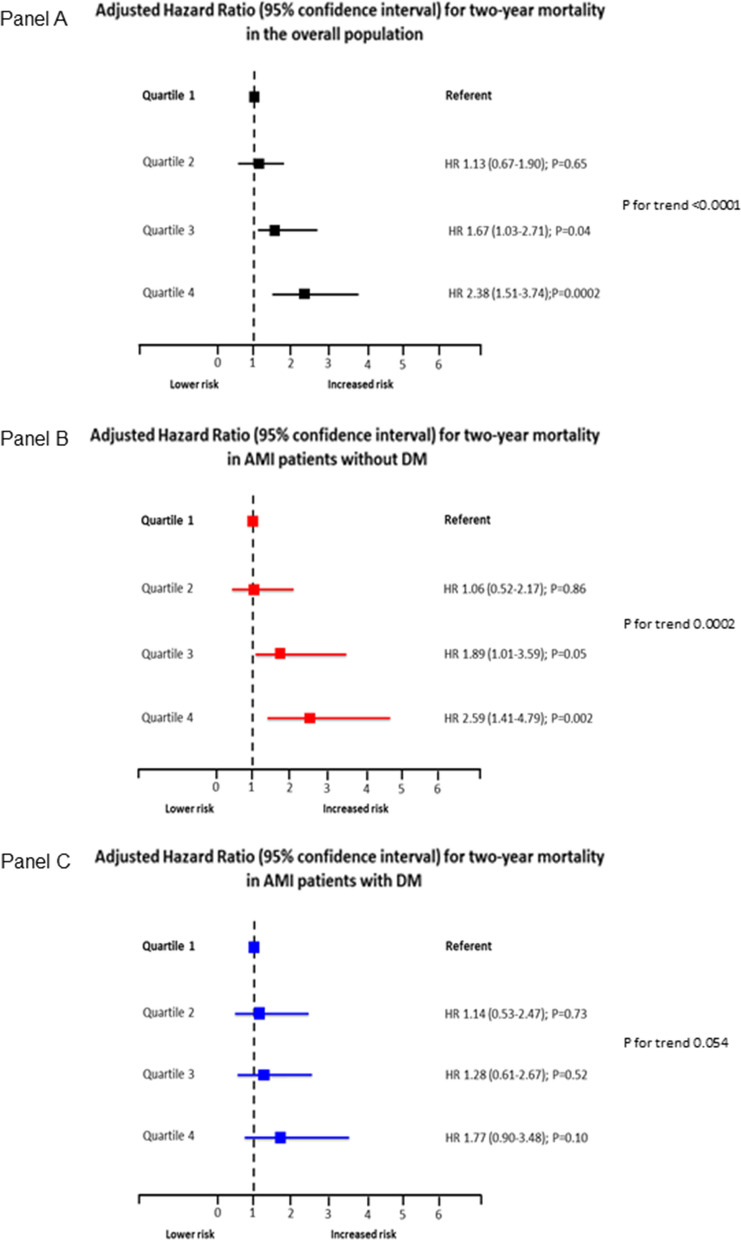
The incidence of the two study endpoints according to hs-CRP quartiles in the overall population, in DM and non-DM patients is shown in Table 3. In the entire study population, the adjusted risk of the primary endpoint increased in parallel with hs-CRP quartiles (Fig. 2; Panel A). However, this trend was more evident in non-DM patients (Fig. 2; Panel B) than in DM patients (Fig. 2; Panel C). A similar behavior was found when two-year mortality was considered (Fig. 3). In line with this result, a significant interaction between DM status and hs-CRP was found for the secondary endpoint (P = 0.02).
Table 3.
Primary and secondary endpoint rates according to high-sensitivity C-reactive protein (hs-CRP) quartiles in the overall study population and in patients with and without diabetes mellitus
| Hs-CRP quartiles | |||||
|---|---|---|---|---|---|
| 1 (< 1.45 mg/L) |
2 (1.45-3.71 mg/L) |
3 (3.72-12.29 mg/L) |
4 (> 12.30 mg/L) |
P for trend | |
| In-hospital clinical combined endpoint, n (%) | |||||
| Overall population | 35 (7%) | 44 (9%) | 68 (13%) | 114 (22%) | < 0.0001 |
| Patients with diabetes mellitus | 14 (14%) | 11 (9%) | 29 (21%) | 51 (27%) | 0.0001 |
| Patients without diabetes mellitus | 21 (5%) | 33 (8%) | 39 (10%) | 63 (19%) | < 0.0001 |
| Two-year mortality, n (%) | |||||
| Overall population | 25 (5%) | 36 (7%) | 49 (9%) | 88 (17%) | < 0.0001 |
| Patients with diabetes mellitus | 11 (11%) | 18 (15%) | 20 (15%) | 41 (22%) | 0.01 |
| Patients without diabetes mellitus | 15 (4%) | 18 (5%) | 29 (8%) | 47 (14%) | <0.0001 |
Fig. 2.
Adjusted odds ratios (OR) and 95% confidence intervals for the primary endpoint according to high-sensitivity C-reactive protein (hs-CRP) level quartiles in the overall study population (Panel A), in patients with diabetes mellitus (DM) (Panel B), and in those without DM (Panel C). Odd ratios and P for trend were adjusted for left ventricular ejection fraction (≤ or > 40%), estimated glomerular filtration rate (≤ or > 60 ml/min/1.73 m2), type of acute myocardial infarction (STEMI vs. NSTEMI), and prior statin use. P for interaction between DM status and hs-CRP = 0.36
Fig. 3.
Adjusted hazard ratios (HR) and 95% confidence intervals for the secondary endpoint according to high-sensitivity C-reactive protein (hs-CRP) level quartiles in the overall study population (Panel A), in patients with diabetes mellitus (DM) (Panel B), and in those without DM (Panel C). Hazard ratios and P for trend were adjusted for left ventricular ejection fraction (≤ or > 40%), estimated glomerular filtration rate (≤ or > 60 ml/min/1.73 m2), type of acute myocardial infarction (STEMI vs. NSTEMI), and prior statin use. P for interaction between DM status and hs-CRP = 0.02
The AUCs for hs-CRP in predicting the primary and secondary endpoints in the entire population were 0.66 (95% CI 0.63–0.70) and 0.66 (95% CI 0.62–0.70), respectively. Again, they were higher in non-DM patients (0.66 [95% CI 0.61–0.70] and 0.67 [95% CI 0.61–0.72]) than in DM patients (0.63 [95% CI 0.58–0.68] and 0.61 [95% CI 0.54–0.67]).
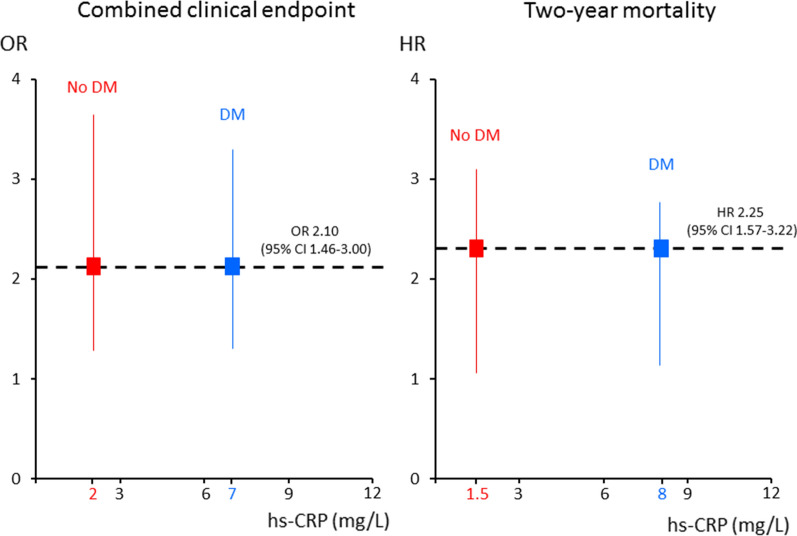
The adjusted OR and HR of the primary and secondary endpoint, respectively, associated with an hs-CRP value ≥ 2 mg/L found in the overall population (Fig. 1) corresponded to higher hs-CRP threshold values in patients with DM than in those without DM (Fig. 4). In parallel, at bootstrap analysis, the hs-CRP cutoff values associated with the primary and secondary endpoint risk in DM patients were higher than those of non-DM patients in 74% and 96% of cases, respectively. When computing adjusted RR of two-year mortality in patients with and without DM, its value increased in both groups in parallel with increasing hs-CRP value. However, RR was consistently higher in non-DM patients for any considered hs-CRP level (Fig. 5).
Fig. 4.
Threshold values of high-sensitivity C-reactive protein (hs-CRP) in patients with and without diabetes mellitus (DM) considered separately, corresponding to the adjusted risk of the primary and secondary endpoints associated with an hs-CRP value ≥ 2 mg/L found in the overall population. OR Odds ratio, HR Hazard ratio, CI Confidence interval
Fig. 5.
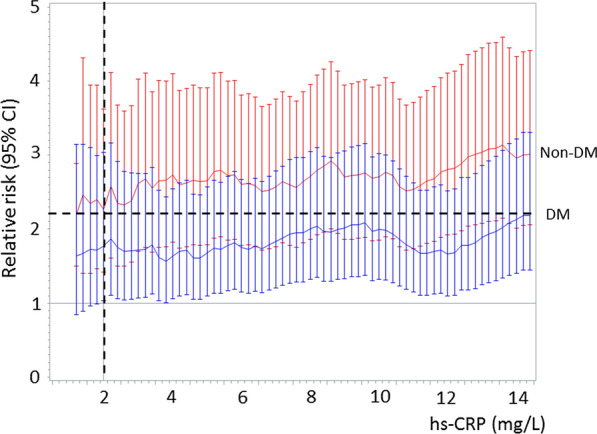
Relative risks and 95% confidence interval (CI) of two-year mortality associated with different high-sensitivity C-reactive protein (hs-CRP) cut-offs in patients with diabetes mellitus (DM) (blue) and in those without DM (red). Relative risk was adjusted for left ventricular ejection fraction (≤ or > 40%), estimated glomerular filtration rate (≤ or > 60 ml/min/1.73 m2), type of acute myocardial infarction (STEMI vs. NSTEMI) and and prior statin use. The vertical dotted line refers to hs-CRP value of 2 mg/L. The horizontal dotted line refers to the RR associated with hs-CRP value of 2 mg/L in non-DM patients
Discussion
This study supports previous evidence showing that hs-CRP measured at hospital admission in AMI patients is a predictor of in-hospital outcome and long-term mortality. This seems to be true for both DM and non-DM patients. However, we demonstrated that the relationship between the outcomes considered in our study and hs-CRP levels is downshifted in DM patients, who show for each hs-CRP value a lower risk than that of non-DM patients. In other words, in DM patients, the hs-CRP values associated to each event risk were higher than those of non-DM patients.
Inflammation and DM in AMI
The involvement of inflammation in atherosclerosis and, consequently, in AMI is well established [4–7], as well as the prognostic usefulness of biomarker surrogates, such as hs-CRP, for predicting the risk of mortality and recurrent events [11–16, 22]. Moreover, observational and randomized studies indicated that cardiovascular benefits are more apparent when systemic inflammation is reduced [23, 24]. In particular, the Aggrastat-to-Zocor (A to Z) trial demonstrated that the clinical outcome of patients with acute coronary syndromes significantly improves when the hs-CRP levels are lowered below 2 mg/L [16]. Diabetes mellitus is a multifactorial metabolic disease and growing evidence shows that it is characterized by a state of sub-clinical inflammation [5], as reflected by chronic high levels of hs-CRP [6]. In AMI, patients with DM show a more severe inflammatory condition than those without DM [25], and this may, at least in part, explain their higher short-term and long-term mortality risk [26, 27]. However, whether hs-CRP during AMI carries a different prognostic relevance in DM and non-DM patients is still a controversial issue. Indeed, on the one hand, previous studies showed that CRP is an independent predictor of mortality after AMI in both DM and non-DM patients [28, 29]. On the other hand, Meisinger et al. [28] found no association between CRP and long-term mortality (median 4 years) after AMI in DM patients. However, these studies were retrospective analyses of registries including old study populations (enrolled between 1998 and 2004), they considered patients with an outdated DM definition [28, 29], and, in one study [29], traditional CRP was assessed. More recently, Xia et al. [30] found that CRP predicts three-year mortality in both DM and non-DM patients with AMI. Yet, in this study, the prognostic relevance of CRP was analyzed according to the CRP median value (8.9 mg/L), a cutoff that may encompass patients with the highest degree of inflammation [30]. Thus, the possible different prognostic impact of hs-CRP in AMI patients with and without DM remains unclear.
In our study, we confirmed the presence of a close association between inflammation and DM status in AMI. Indeed, DM patients were more likely to have admission hs-CRP levels ≥ 2 mg/L and had a higher median hs-CRP value than non-DM patients. Moreover, both inflammation and DM status, considered separately, were predictors of in-hospital outcome and two-year mortality, even after adjustment for major confounders. However, when we investigated the relationship between inflammation and outcomes, hs-CRP showed a different behavior in DM and in non-DM patients. In particular, the adjusted risk of the primary and secondary endpoints increased in parallel with hs-CRP quartiles in both groups, but with a more evident trend in non-DM patients. Notably, in the overall population, an hs-CRP value ≥ 2 mg/L was associated with an almost two-fold higher risk of both endpoints. This same risk corresponded to higher hs-CRP values in DM patients, when compared to non-DM patients, thus suggesting that the prognostic relevance of inflammation is maintained also in DM patients but it is shifted towards higher hs-CRP levels. To the best of our knowledge, this is a novel finding, which, if confirmed in future studies, could pave the way for prognostic stratification and intervention strategies tailored according to DM status.
The mechanisms underlying the different prognostic behavior of hs-CRP in DM and non-DM patients are beyond the purpose of the present analysis. However, the following hypothesis can be proposed. In AMI patients, admission hs-CRP level may be considered the result of a variable combination of chronic and acute inflammation. Thus, high hs-CRP levels at hospital admission may not necessarily represent only the inflammatory response associated with AMI severity. Given the well-established association between DM and inflammation, the contribution of chronic inflammation to hs-CRP levels in AMI patients is possibly more relevant in DM than in non-DM patients. Consistently with this theory, a similar hs-TnI peak value, an estimate of myocardial infarct size, was observed in our study in DM and non-DM patients, although the median hs-CRP level was significantly higher in the former group. Moreover, the correlation between hs-CRP levels and hs-TnI peak value was closer in non-DM than in DM patients.
Another intriguing issue is represented by the mechanisms underlying the association between hs-CRP and in-hospital outcome in AMI. In this regard, there is growing evidence that inflammation in AMI is not only a marker of AMI severity but it may directly exacerbate the cardiac dysfunction [31–33]. Indeed, in conditions characterized by acute systemic inflammation—such as severe burn, trauma, or sepsis—cardiac cell death is rare but reversible cardiac myocyte injury often occurs resulting in a transient depression of myocardial contractility [31–33]. Notably, the most important mediators of the inflammatory process, like tumor necrosis factor-α, interleukin-1β, and interleukin-6, have been shown to have a negative inotropic effect on cardiac contractility [31, 32]. Moreover, an association between elevation of inflammatory markers and myocardial reperfusion injury has been reported in AMI [34]. On this account, we considered a combined in-hospital clinical endpoint including acute pulmonary edema, cardiogenic shock, and death, which are clinical equivalents of acute ventricular dysfunction.
Study clinical implications
Our study may have some potential clinical implications. Firstly, in AMI patients, hs-CRP allows physicians to identify high-risk patients. This is true also for DM patients, in whom, however, a higher hs-CRP threshold than that usually considered (2 mg/L) should be identified to improve risk stratification. This concept is further suggested by the fact that, in our study population, a significant interaction was found between DM status and hs-CRP when long-term mortality was considered. Moreover, the RR of two-year mortality was constantly lower in patients with DM than in those without DM at each given hs-CRP level. For instance, the two-year mortality RR of a non-DM patient with hs-CRP level of 2 mg/L was similar to that of a DM patient with hs-CRP level of 14 mg/L (Fig. 5). Secondly, as hs-CRP has been recently considered a potential therapeutic target in AMI, DM status should be taken into account when anti-inflammatory therapeutic strategies are investigated. The Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) trial showed that, among patients with prior AMI and hs-CRP ≥ 2 mg/L, treatment with a monoclonal antibody targeting interleukin-1β is associated with fewer cardiovascular events [23]. However, in the CANTOS trial, the beneficial effects, in terms of cardiovascular endpoints, were mainly observed in non-DM patients, with a non-significant risk reduction in those with DM [24]. This highlights the possible need of a different hs-CRP cutoff value for the identification of high-risk AMI patients with DM who may benefit the most from an anti-inflammatory therapeutic strategy. Novel therapeutic approaches aiming at reducing hs-CRP levels during AMI are also under investigation, and preliminary experimental and clinical data are being reported on the use of apheresis in this clinical setting [35, 36]. This strategy demonstrated to rapidly and safely lower hs-CRP levels by about 50%, independently of the initial concentration [35]. Interestingly, this reduction was associated with a smaller infarct size in animal models [36].
Study strengths and limitations
The strengths of our study include its prospective design, a well-characterized population, and a special focus on the relationship between inflammation and DM status in AMI. However, some limitations warrant mention. Firstly, we evaluated an AMI population admitted to a single center and treated, in most cases, with PCI. As this therapeutic strategy may have influenced the results of our study, the overall applicability of our findings to AMI patients not undergoing coronary revascularization needs to be clarified. Moreover, the promptness, extent, and efficacy of myocardial revascularization was not assessed as a confounder event. Secondly, because of the observational nature of the study, a cause-effect relationship between hs-CRP and outcomes cannot be established. Moreover, we did not evaluate the effectiveness of and the adherence to pharmacological treatment, in particular of lipid- and glucose-lowering therapies, during follow-up. Indeed, previous studies reported that the prognostic effect of admission hs-CRP is attenuated in high-intensity statin users [37] and in patients with optimized glycometabolic control [38]. Thirdly, the association between hs-CRP levels at admission and the duration and treatment of DM was not investigated, and it should be considered as a possible bias. Fourthly, we measured only hs-CRP; however, it is possible that other inflammation indexes, such as the CRP to albumin ratio, may be more accurate in predicting outcomes in AMI patients [39, 40]. Fifthly, we measured hs-CRP levels only at admission. As the inflammatory response in AMI begins within hours and peaks after several days [41], hs-CRP levels at other time points might better reflect the magnitude of the acute inflammatory process. Finally, several potential confounding factors associated with chronic inflammation, such as thickened epicardial adipose tissue [42] and fibrinogen concentration [43], were not evaluated in our study.
Conclusions
The results of this study show that hs-CRP level measured at hospital admission predicts in-hospital outcome and two-year mortality in AMI patients with and without DM. However, in patients with DM, the same risk of developing events as in non-DM patients is associated to higher hs-CRP levels.
Acknowledgements
Not applicable.
Abbrevations
- AMI
Acute myocardial infarction
- CAD
Coronary artery disease
- CI
Confidence intervals
- CRP
C-reactive protein
- DM
Diabetes mellitus
- OR
Odds ratio
- hs-CRP
High-sensitivity-C-reactive protein
- HR
Hazard ratio
- LVEF
Left ventricular ejection fraction
- NSTEMI
non-ST-elevation myocardial infarction
- PCI
Percutaneous coronary intervention
- ROC
Receiver-operating characteristics
- RR
Relative risk
- STEMI
ST-elevation myocardial infarction
Authors’ contributions
GM, CL and ALB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CL, NC, GM, ALB. Acquisition of data: SG, JC, VM, MM, MR, DR, MLB, MR, KC. Analysis and interpretation of data: GM, NC, JC, ALB. Drafting the manuscript: CL, GM, NC. Critical revision of the manuscript for important intellectual content: PA, SG, ALB. Statistical analysis: AB, NC, FV. Administrative, technical, or material support: GM. Study supervision: GM, PA, ALB. All authors read and approved the final manuscript.
Funding
This work was supported by the Centro Cardiologico Monzino, IRCCS, Milan, Italy. The study’s sponsor had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available, as per internal protocol, but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
the Ethics Committee (n. R520-CCM549) approved the study as a prospective cohort study.
Consent for publication
not applicable.
Competing interests
none.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brener SJ, Mehran R, Dressler O, Cristea E, Stone GW. Diabetes mellitus, myocardial reperfusion, and outcome in patients with acute ST-elevation myocardial infarction treated with primary angioplasty (from HORIZONS AMI) Am J Cardiol. 2012;109:1111–1116. doi: 10.1016/j.amjcard.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014–1019. doi: 10.1161/01.CIR.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 3.Marenzi G, Cosentino N, Genovese S, Campodonico J, De Metrio M, Rondinelli M, Cornara S, Somaschini A, Camporotondo R, Demarchi A, Milazzo V, Moltrasio M, Rubino M, Marana I, Grazi M, Lauri G, Bonomi A, Veglia F, De Ferrari GM, Bartorelli AL. Reduced cardio-renal function accounts for most of the in-hospital morbidity and mortality risk among patients with type 2 diabetes undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Diab Care. 2019;42:1305–1311. doi: 10.2337/dc19-0047. [DOI] [PubMed] [Google Scholar]
- 4.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- 5.Pitsavos C, Tampourlou M, Panagiotakos DB, Skoumas Y, Chrysohoou C, Nomikos T, Stefanadis C. Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA Study. Rev Diabet Stud. 2007;4:98–104. doi: 10.1900/RDS.2007.4.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odegaard AO, Jacobs DR, Jr, Sanchez OA, Goff DC, Jr, Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15:51. doi: 10.1186/s12933-016-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 8.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–1357. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397–408. doi: 10.1016/j.jacc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 12.Fu EL, Franko MA, Obergfell A, Dekker FW, Gabrielsen A, Jernberg T, Carrero JJ. High-sensitivity C-reactive protein and the risk of chronic kidney disease progression or acute kidney injury in post-myocardial infarction patients. Am Heart J. 2019;216:20–29. doi: 10.1016/j.ahj.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino N, Genovese S, Campodonico J, Bonomi A, Lucci C, Milazzo V, Moltrasio M, Biondi ML, Riggio D, Veglia F, Ceriani R, Celentano K, De Metrio M, Rubino M, Bartorelli AL, Marenzi G. High-sensitivity C-reactive protein and acute kidney injury in patients with acute myocardial infarction: a prospective observational study. J Clin Med. 2019;8:2192. doi: 10.3390/jcm8122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zebrack JS, Anderson JL, Maycock CA, Horne BD, Bair TL, Muhlestein JB, Intermountain Heart Collaborative (IHC) Study Group Usefulness of high-sensitivity C-reactive protein in predicting long-term risk of death or acute myocardial infarction in patients with unstable or stable angina pectoris or acute myocardial infarction. Am J Cardiol. 2002;89:145–149. doi: 10.1016/S0002-9149(01)02190-7. [DOI] [PubMed] [Google Scholar]
- 15.Mani P, Puri R, Schwartz GG, Nissen SE, Shao M, Kastelein JJP, Menon V, Lincoff AM, Nicholls SJ. Association of initial and serial C-reactive protein levels with adverse cardiovascular events and death after acute coronary syndrome: a secondary analysis of the VISTA-16 trial. JAMA Cardiol. 2019;4:314–320. doi: 10.1001/jamacardio.2019.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiviott SD, de Lemos JA, Cannon CP, Blazing M, Murphy SA, McCabe CH, Califf R, Braunwald E. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406–1414. doi: 10.1161/CIRCULATIONAHA.105.586347. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020. 43 (Suppl 1):S14-S31. [DOI] [PubMed]
- 18.Podmore C, Meidtner K, Schulze MB, Scott RA, Ramond A, Butterworth AS, Di Angelantonio E, Danesh J, Arriola L, Barricarte A, Boeing H, Clavel-Chapelon F, Cross AJ, Dahm CC, Fagherazzi G, Franks PW, Gavrila D, Grioni S, Gunter MJ, Gusto G, Jakszyn P, Katzke V, Key TJ, Kühn T, Mattiello A, Nilsson PM, Olsen A, Overvad K, Palli D, Quirós JR, Rolandsson O, Sacerdote C, Sánchez-Cantalejo E, Slimani N, Sluijs I, Spijkerman AM, Tjonneland A, Tumino R, van der A DL, van der Schouw YT, Feskens EJ, Forouhi NG, Sharp SJ, Riboli E, Langenberg C, Wareham NJ. The association of multiple biomarkers of iron metabolism and type 2 diabetes—the EPIC-InterAct Study. Diabetes Care. 2016. 39:572-581. [DOI] [PMC free article] [PubMed]
- 19.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;2019(40):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 20.Lehto M, Snapinn S, Dickstein K, Swedberg K, Nieminen MS, OPTIMAAL investigators Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: the OPTIMAAL experience. Eur Heart J. 2005;26:350–356. doi: 10.1093/eurheartj/ehi064. [DOI] [PubMed] [Google Scholar]
- 21.Diamantis E, Kyriakos G, Quiles-Sanchez LV, Farmaki P, Troupis T. The anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr Cardiol Rev. 2017;13:209–216. doi: 10.2174/1573403X13666170426104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol. 2006;47:962–968. doi: 10.1016/j.jacc.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017. 377:1119-1131. [DOI] [PubMed]
- 24.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomized controlled trial. Lancet. 2018. 391:319-328. [DOI] [PubMed]
- 25.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diab. 2014;5:444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, Rubino M, Campodonico J, Moltrasio M, Marana I, Grazi M, Lauri G, Bonomi A, Veglia F, Manfrini R, Bartorelli AL. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diab Care. 2018;41:847–853. doi: 10.2337/dc17-1732. [DOI] [PubMed] [Google Scholar]
- 27.Milazzo V, Cosentino N, Genovese S, Campodonico J, Mazza M, De Metrio M, Marenzi G. Diabetes mellitus and acute myocardial infarction: impact on short and long-term mortality. Adv Exp Med Biol. 2020. [DOI] [PubMed]
- 28.MONICA/KORA Myocardial Infarction Registry, Meisinger C, Heier M, von Scheidt W, Kuch B. Admission C reactive protein and short- as well as long-term mortality in diabetic versus nondiabetic patients with incident myocardial infarction. Clin Res Cardiol. 2010. 99:817-823. [DOI] [PubMed]
- 29.Otter W, Winter M, Doering W, Standl E, Schnell O. C-reactive protein in diabetic and nondiabetic patients with acute myocardial infarction. Diabetes Care. 2007;30:3080–3082. doi: 10.2337/dc07-1020. [DOI] [PubMed] [Google Scholar]
- 30.Xia M, Zhang C, Gu J, et al. Impact of C-reactive protein on long-term mortality in acute myocardial infarction patients with diabetes and those without. Clin Chim Acta. 2018;480:220–224. doi: 10.1016/j.cca.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Rudiger A, Singer M. The heart in sepsis: from basic mechanisms to clinical management. Curr Vasc Pharmacol. 2013;11:187–195. [PubMed] [Google Scholar]
- 32.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walley KR. Sepsis-induced myocardial dysfunction. Curr Opin Crit Care. 2018;24:292–299. doi: 10.1097/MCC.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 34.Niccoli G, Lanza GA, Spaziani C, Altamura L, Romagnoli E, Leone AM, Fusco B, Trani C, Burzotta F, Mazzari MA, Mongiardo R, Biasucci LM, Rebuzzi AG, Crea F. Baseline systemic inflammatory status and no-reflow phenomenon after percutaneous coronary angioplasty for acute myocardial infarction. Int J Cardiol. 2007;117:306–311. doi: 10.1016/j.ijcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Ries W, Heigl F, Garlichs C, Sheriff A, Torzewski J. Selective C-Reactive Protein-Apheresis in Patients. Ther Apher Dial. 2019. [DOI] [PubMed]
- 36.Sheriff A, Schindler R, Vogt B, Abdel-Aty H, Unger JK, Bock C, Gebauer F, Slagman A, Jerichow T, Mans D, Yapici G, Janelt G, Schröder M, Kunze R, Möckel M. Selective apheresis of C reactive protein: a new therapeutic option in myocardial infarction? J Clin Apher. 2015;30:15–21. doi: 10.1002/jca.21344. [DOI] [PubMed] [Google Scholar]
- 37.Kang DO, Park Y, Seo JH, Jeong MH, Chae SC, Ahn TH, Jang WY, Kim W, Park EJ, Choi BG, Na JO, Choi CU, Kim EJ, Rha SW, Park CG, Seo HS, KAMIR-NIH Registry Investigators Time-dependent prognostic effect of high sensitivity C-reactive protein with statin therapy in acute myocardial infarction. J Cardiol. 2019;74:74–83. doi: 10.1016/j.jjcc.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Winzap P, Davies A, Klingenberg R, Obeid S, Roffi M, Mach F, Räber L, Windecker S, Templin C, Nietlispach F, Nanchen D, Gencer B, Muller O, Matter CM, von Eckardstein A, Lüscher TF. Diabetes and baseline glucose are associated with inflammation, left ventricular function and short- and long-term outcome in acute coronary syndromes: role of the novel biomarker Cyr 61. Cardiovasc Diab. 2019;18:142. doi: 10.1186/s12933-019-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karabağ Y, Çağdaş M, Rencuzogullari I, Karakoyun S, Artaç İ, İliş D, Yesin M, Çiftçi H, Erdoğdu HI, Tanboğa IH. The C-reactive protein to albumin ratio predicts acute kidney injury in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Lung Circ. 2019;28:1638–1645. doi: 10.1016/j.hlc.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Ammirati E, Cannistraci CV, Cristell NA, Vecchio V, Palini AG, Tornvall P, Paganoni AM, Miendlarzewska EA, Sangalli LM, Monello A, Pernow J, Björnstedt Bennermo M, Marenzi G, Hu D, Uren NG, Cianflone D, Ravasi T, Manfredi AA, Maseri A. Identification and predictive value of interleukin-6 + interleukin-10 + and interleukin-6- interleukin-10 + cytokine patterns in ST-elevation acute myocardial infarction. Circ Res. 2012;111:1336–1348. doi: 10.1161/CIRCRESAHA.111.262477. [DOI] [PubMed] [Google Scholar]
- 41.Cristell N, Cianflone D, Durante A, Ammirati E, Vanuzzo D, Banfi M, Calori G, Latib A, Crea F, Marenzi G, De Metrio M, Moretti L, Li H, Uren NG, Hu D, Maseri A; FAMI Study Investigators. High-sensitivity C-reactive protein is within normal levels at the very onset of first ST-segment elevation acute myocardial infarction in 41% of cases: a multiethnic case-control study. J Am Coll Cardiol. 2011. 58:2654-2661. [DOI] [PubMed]
- 42.Cho DH, Joo HJ, Kim MN, Lim DS, Shim WJ, Park SM. Association between epicardial adipose tissue, high-sensitivity C-reactive protein and myocardial dysfunction in middle-aged men with suspected metabolic syndrome. Cardiovasc Diab. 2018;17:95. doi: 10.1186/s12933-018-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Xu C, Liu J, Bai X, Li R, Wang L, Zhou J, Wu Y, Yuan Z. Baseline plasma fibrinogen is associated with haemoglobin A1c and 2-year major adverse cardiovascular events following percutaneous coronary intervention in patients with acute coronary syndrome: a single-centre, prospective cohort study. Cardiovasc Diabetol. 2019;18:52. doi: 10.1186/s12933-019-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available, as per internal protocol, but are available from the corresponding author on reasonable request.