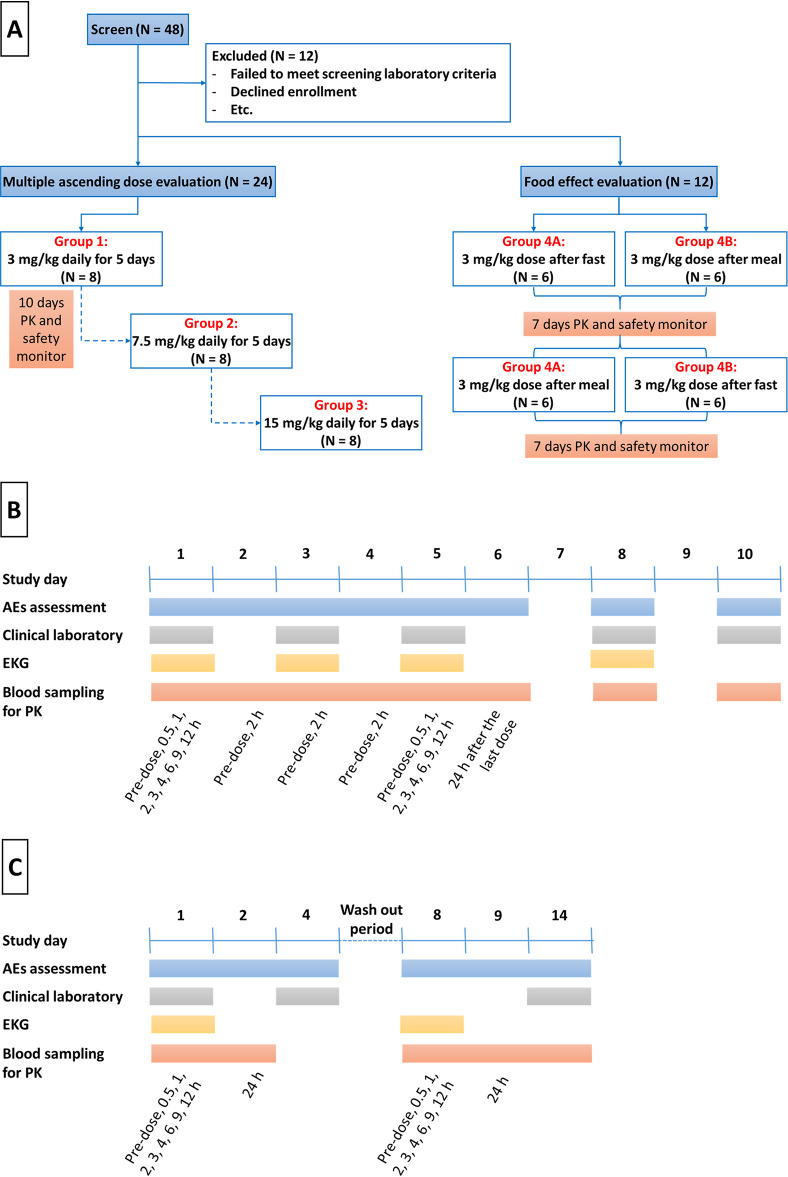
FIG 5.
(A) Schematic diagram of the multiple ascending dose study and the food effect study. (B and C) Schedules of blood sample collection for pharmacokinetics assessment and adverse event assessment for subjects participating in the multiple ascending dose evaluation (B) and the food effect evaluation (C).