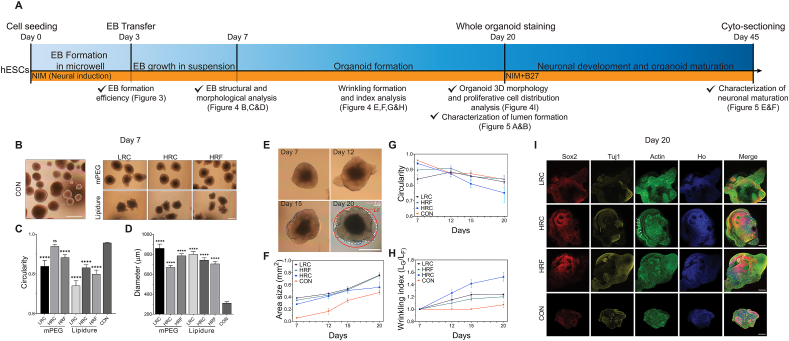
Fig. 4.
Characterization of EB and organoid formation. A) Timeline illustrated a multi-stage culturing process, including EB formation in microwells, EB growth in suspension, organoid formation, and organoid maturation. Major experiments and procedures were labeled above the timeline, along with the days. Major analyses and associated figures in this paper were labeled beneath the timeline. The cell culture medium condition was tagged in the yellow stripe. B) Representative bright-field images of EBs on day 7 of the control group (CON) and microwell groups (with respect to three geometries and two coating conditions). C) EB circularity on day 7. The mPEG-HRC was not significantly different from CON, while other microwell groups were significantly lower. D) EB diameter on day 7. All groups were significantly larger than CON group in diameter. E) Bright-field images of HRC-derived organoids from day 7 to day 20 exemplified the organoid growth and wrinkling/folding formation. The bottom right image illustrated how LG and LF were measured for the determination of the wrinkling index. F) Area size as a function of time in LRC, HRC, HRF, and CON. All showed an increasing trend, with all microwell groups significantly larger than CON. G) Circularity as a function of time in LRC, HRC, HRF, and CON. The circularity of all groups declined after day 7 or 12 at various speeds, with CON being the slowest and HRC being the fastest. The HRC group achieved the lowest circularity on day 20. H) Wrinkling index as a function of time in LRC, HRC, HRF, and CON. Wrinkling index of all microwell groups increased since day 7 at various speeds, with HRC group being the fastest and the highest. In contrast, CON group stayed un-wrinkled (wrinkle index ~ 1.0) until day 20, when the wrinkle index increased slightly. I) Immunocytochemistry of whole-mount organoids on day 20 revealed the overall cell organization for each microwell group and CON. Sox2, Tuj1, Actin, and Hoechst (Ho) were used to mark stem cells, differentiated neurons, cytoskeleton (neurites), and nuclei (all cell types). The location of wrinkling grooves was marked with white arrows. Note, all microwells used in E)-I) were coated with mPEG for maximum performance. Scale Bars (B, E): 500 μm; (I) 200 μm. (C, D) Every condition was statistically compared to CON group using Student's t-test ****P < 0.0001; ns, no significance. All data were presented as the mean ± SEM.