Figure 1.
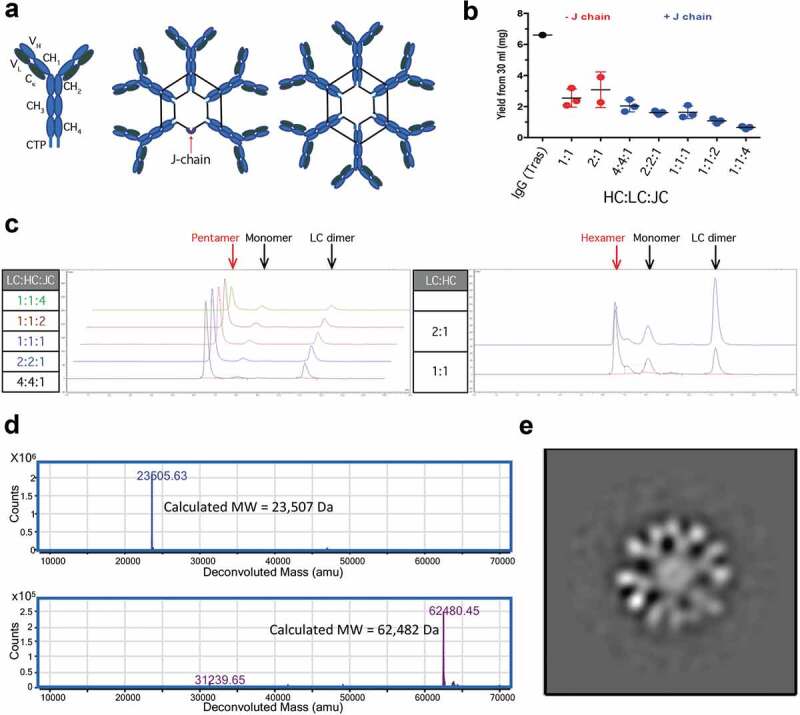
Characterization of recombinant IgMs. Structural representations of IgM ‘monomers’, pentamers, and hexamers. Black lines joining HC represent disulfide bonds (a). 30 ml of Expi293 cells were transiently transfected with various ratios of hIgM HC:LC:JC incubated for 7 d and partially purified using CaptoL resin. Samples were analyzed for total protein by A280 using trastuzumab (Tras) as a positive control (b) and for homogeneity using SEC (c). Human IgM hexamers were purified via two-step purifications. Purified hexamer was reduced and deglycosylated prior to analysis by LC-MS/MS. Extracted ion chromatograms are shown for LC (top) and heavy chain (bottom) (d). Negative Staining TEM characterization of hIgM hexamer reveals the anticipated six-fold symmetry (e).
