Fig. 2. Monomeric Skp is intrinsically disordered.
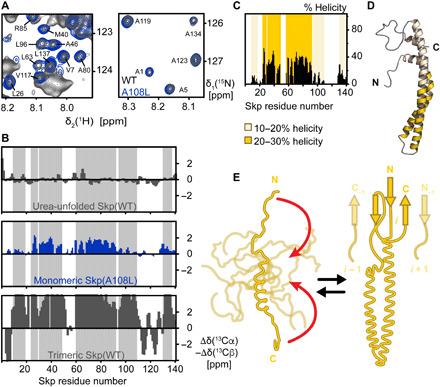
(A) Sections of 2D [15N,1H]-TROSY spectra of [U-2H,15N] Skp(WT) (dark gray) and Skp(A108L) (blue) at a concentration of 1 mM and 37°C in NMR buffer (20 mM MES, pH 6.5, and 150 mM NaCl). NMR signals of the monomeric state of Skp(WT) are overlaying with the one from Skp(A108L). The assignments of the overlapping NMR signals of the monomeric state are indicated in the panel. (B) Residue-specific secondary backbone chemical shifts of Skp(WT) in 8 M urea solution, Skp(A108L) in its monomeric form, and Skp(WT) in its trimeric form. Positive and negative values indicate α-helical and β-sheet secondary structure elements, respectively. The gray-shaded area indicates the positions of helices in the Skp trimer. (C) Percentage of helical population in the conformational ensemble of the Skp monomer. Helical regions with 10 to 20% helicity or 20 to 30% helicity are highlighted with light or dark yellow, respectively. (D) Structural model of the Skp monomer. On a configuration of Skp with α-helices formed, the degree of residual helical population present in the conformational ensemble is indicated. The large majority of monomeric Skp is disordered. (E) Schematic model of coupled oligomerization and folding mechanism of Skp. Monomeric Skp explores an ensemble of conformations with a low propensity for the formation of the arm α-helices. The formation of the oligomerization interface brings the N and C termini together (red arrows), thus stabilizing the coiled-coil structure of the α-helical arms.
