Figure 1.
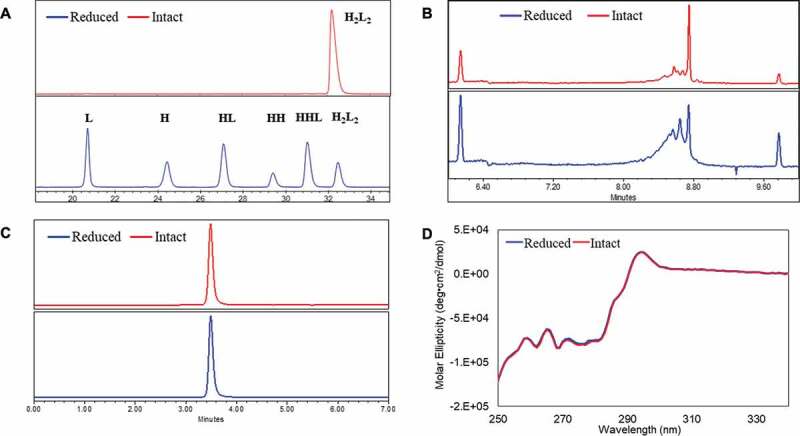
Conformations between intact and partially reduced monoclonal antibody (mAb), mAb-1 (IgG1). (a) Non-reduced CE-SDS analysis showed H2L2, HHL, HH, HL, H, and L for the reduced sample; (b) icIEF analysis showed different charge profiles between the reduced and intact mAb samples; (c) SEC analysis showed identical native sizes for the reduced and intact mAb samples; and (d) near-UV circular dichroism analysis showed identical high-order structures for the reduced and intact mAb samples. The intact mAb was defined with ≥ 90% purity based on non-reduced CE-SDS measurement and partially reduced mAbs was defined with < 90% purity. Analytical measurements details were documented in Material and Methods. H2L2: intact mAb, L: light chain, H: heavy chain, HH: heavy-heavy fragment, HL: halfmer, HHL: heavy-heavy-light fragment.
