Figure 3. Individually and collectively migrating neural crest cells utilize many of the same cellular mechanisms for migration.
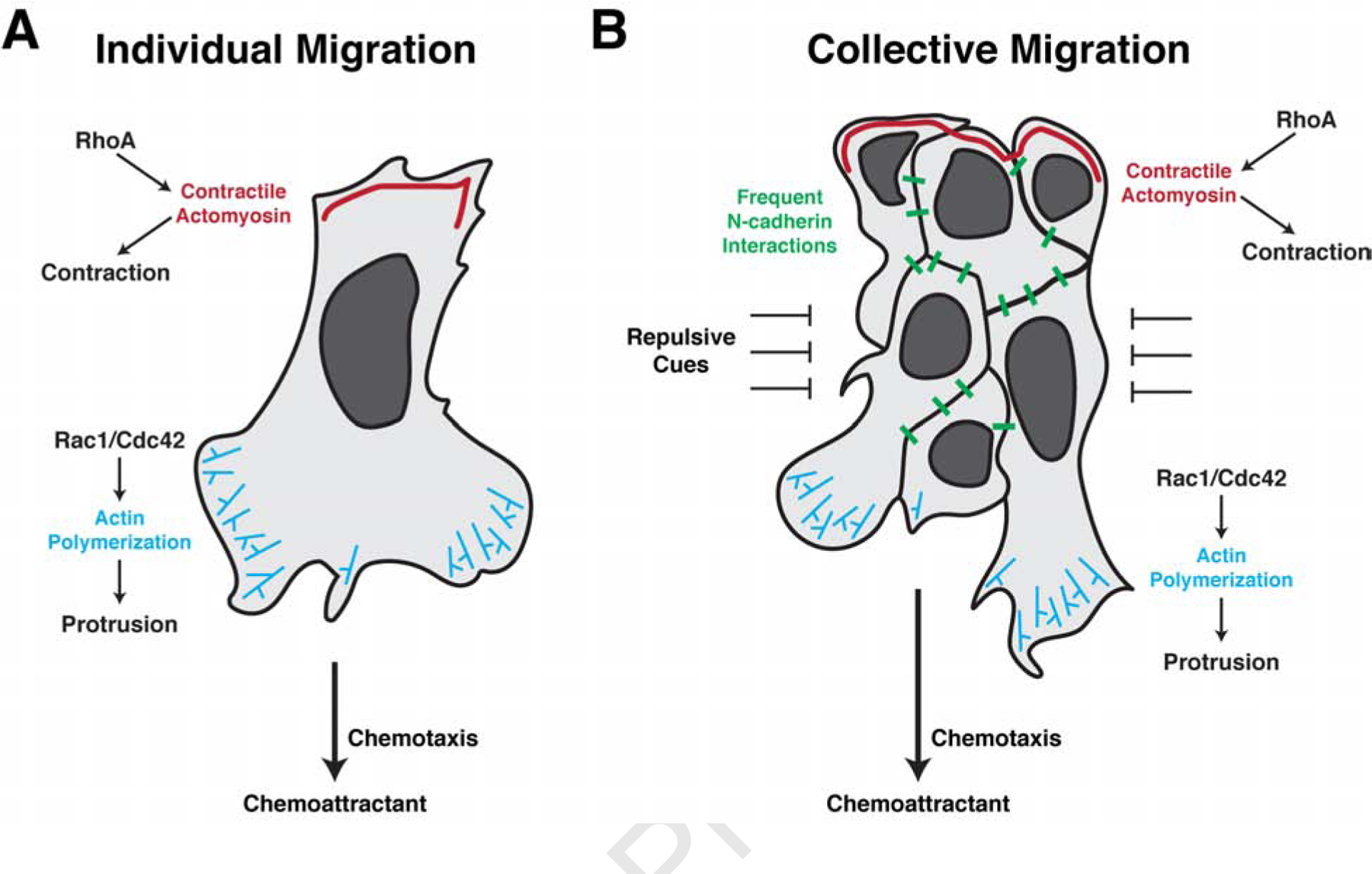
(a) Individually migrating neural crest cells undergo chemotaxis to maintain directional movement. Binding of the chemokine to its receptors on the cell surface polarize the activity of the small GTPases Rac1, Cdc42, and RhoA. At the leading edge, Rac1/Cdc42 activation promotes actin polymerization to extend lamellipodia and filopodia, allowing for new adhesions with the neighboring environment. RhoA activity is restricted to the trailing edge where it controls myosin phosphorylation and actomyosin contractions, propelling the cell forward. Infrequent N-cadherin interactions between individually migrating cells further shape their directionality. (b) In collectively migrating neural crest cells, chemotaxis similarly promotes leading edge Rac1/Cdc42 activation and actin polymerization in the leading cells. Trailing cells, however, activate RhoA to mediate supracellular contractions to move the migratory cluster forward. Simultaneously, frequent N-cadherin interactions maintain cluster spreading and fluidity through contact inhibition of locomotion. Collectively migrating cells are tightly confined by adjacent repulsive cues which aid to maintain cohesiveness of the migratory tissue.
