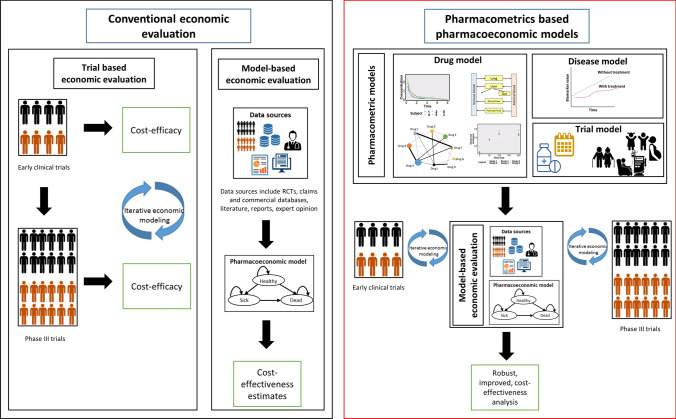
Fig. 1.
Economic evaluations conducted alongside clinical trials provide estimates of cost-efficacy. Conventional pharmacoeconomic analysis of new drugs are usually performed at the end of phase 3 trials. At this stage, non-establishment of cost-effectiveness might delay the approval and marketing of drugs. Early cost-effectiveness analysis, informed by comparative effectiveness evidence generated from pharmacometric models conducted across the drug development pathway can be informed by the well-established MBDD framework. Drug models include the characterization of concentration-time-effect relationship. Disease progression models describe the relationship between time course of disease and disease-specific biomarkers. Trial model describes protocol-deviations such as medication non-adherence and special populations. These analyses can be used to 1) provide early estimates of cost-effectiveness to support strategic R&D decisions of pipeline drugs (e.g., early termination of uneconomic product, resource utilization) and pricing decisions (e.g., consideration of benefit in value-based pricing). Additionally, they can help model long-term outcomes from surrogate end-points, thus predicting formulations, dosing strategies and patient sub-groups that are likely to show cost-effectiveness, especially in scenarios where conducting clinical trials is not possible. 2) Design efficient and more informative phase 3 trials. 3) Assess the impact of real-world scenarios such as non-adherence, dose adaptation in response to toxicity or public health care utilization patterns and outcomes