Figure 2.
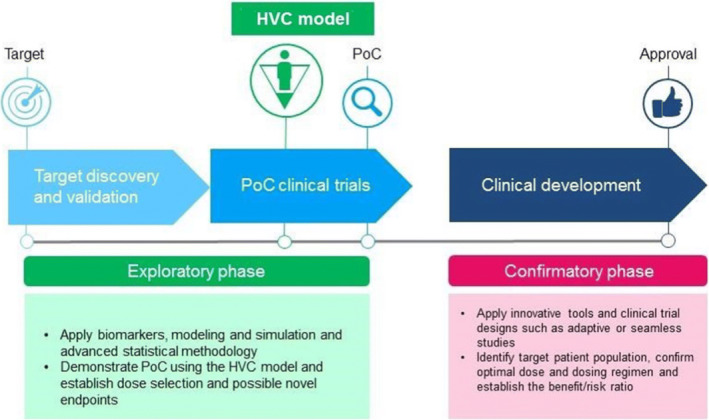
The role of the HVC model in the clinical development pathway. Short duration proof‐of‐concept studies, which incorporate the HVC model, typically include small numbers of subjects. The resulting safety and, particularly, efficacy data can more accurately guide decisions on whether to expose a larger number of subjects to promising candidate therapeutics in community‐based 14 field studies than conventional phase 1 safety data alone might otherwise 12
