Summary
The receptor tyrosine kinase AXL is associated with epithelial plasticity in several solid tumors including breast cancer and AXL-targeting agents are currently in clinical trials. We hypothesized that AXL is a driver of stemness traits in cancer by co-option of a regulatory function normally reserved for stem cells. AXL-expressing cells in human mammary epithelial ducts co-expressed markers associated with multipotency, and AXL inhibition abolished colony formation and self-maintenance activities while promoting terminal differentiation in vitro. Axl-null mice did not exhibit a strong developmental phenotype, but enrichment of Axl+ cells was required for mouse mammary gland reconstitution upon transplantation, and Axl-null mice had reduced incidence of Wnt1-driven mammary tumors. An AXL-dependent gene signature is a feature of transcriptomes in basal breast cancers and reduced patient survival irrespective of subtype. Our interpretation is that AXL regulates access to epithelial plasticity programs in MaSCs and, when co-opted, maintains acquired stemness in breast cancer cells.
Subject Areas: Cell Biology, Stem Cells Research, Cancer
Graphical Abstract
Highlights
-
•
AXL + mammary epithelial cells have multipotent activity conserved in women and mice
-
•
AXL allows accesses to epithelial-to-mesenchymal transition genes and prevents differentiation into luminal cells
-
•
Deletion of Axl reduced incidence of Wnt1-driven tumors in mice
-
•
Provides a rationale explaining the advantage to cancer cells that co-opt AXL signaling
Cell Biology; Stem Cells Research; Cancer
Introduction
Phenotypic plasticity, the capacity of a single genotype to exhibit variable phenotypes in different environments, is a key feature of epithelial homeostasis. The multi-lineage potential of epithelial stem cells is thought to be maintained by specific niche microenvironments and elicited by regenerative cues from tissue wounding and inflammation (Blanpain and Fuchs, 2014). Co-option of these homeostatic mechanisms by carcinoma cells facilitates transition between stem-like mesenchymal and differentiated epithelial states in response to tumor microenvironment dynamics and therapeutic challenge and is associated with poor clinical outcome (Nieto et al., 2016).
The adult human mammary gland is a bilayer epithelium with basal-located myoepithelial (MEP) cells that surround luminal epithelial cells (LEPs) that are thought to be maintained by mammary stem cells (MaSCs), which continue to elude a consensus definition (Fridriksdottir et al., 2017; Petersen and Polyak, 2010; Villadsen et al., 2007; Eirew et al., 2008). The breast epithelium exhibits remarkable organ-scale remodeling during puberty and multiple lactation cycles that requires a renewable reservoir of stem and committed progenitor cells (Visvader and Stingl, 2014). Stem cell-related gene expression programs are utilized by breast cancer cells during malignant progression – here also referred to as co-option (Lawson et al., 2015; Billaud and Santoro, 2011). Transcription factors (e.g. SNAI1/2) that regulate the epithelial-to-mesenchymal transition (EMT) gene program during early development influence MaSC state transitions, and their dysregulation causes luminal compartment expansion (Phillips et al., 2014; Ye et al., 2015). The AXL receptor tyrosine kinase (RTK) is associated with malignant progression and poor patient survival in several malignancies including breast cancer (Davidsen et al., 2017). AXL is activated by a single ligand, GAS6, that activates a unique RTK signaling network in cancer cells (Meyer et al., 2013). AXL expression is correlated with epithelial-mesenchymal transition, immune evasion, increased metastatic potential, as well as therapeutic resistance in several tumor types (Gjerdrum et al., 2010; Ludwig et al., 2018; Zhang et al., 2012; Antony et al., 2016; Lotsberg et al., 2020; Terry et al., 2019). AXL is therefore an important therapeutic target, and AXL kinase inhibitors are currently in clinical trials (Davidsen et al., 2017).
In contrast to the prominence of AXL in cancer progression, the role of AXL signaling in normal physiology is comparatively unknown, limiting our understanding of the consequences of AXL activation in malignant progression. We hypothesize that AXL is a gatekeeper to the signaling cascades and gene programs that enable epithelial plasticity, and that its principle role in normal epithelia is to regulate access to programs that are permissive for stem or progenitor cell states. Herein we identify AXL as a conserved mediator that governs human and mouse MaSC activity, providing a conserved marker of adult stem cells that is correlated with epithelial plasticity.
Results
AXL Expression Is a Feature of Rare Adult Human Breast Epithelial Cells
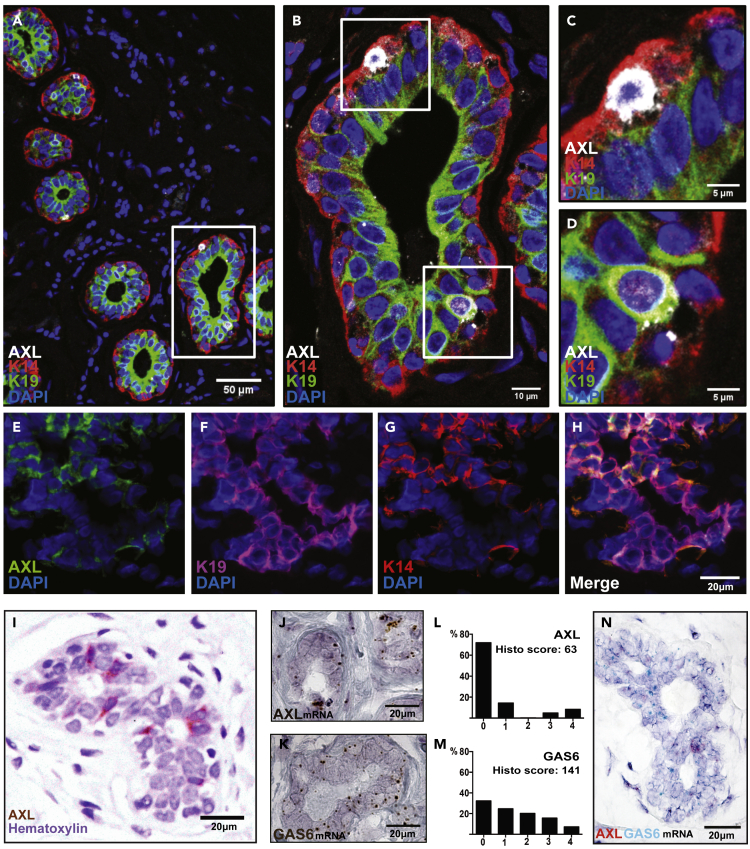
AXL knockout mice are viable and do not show a developmental phenotype (Lu and Lemke, 2001); thus there is no evidence linking AXL to developmental EMT. This prompted us to investigate whether AXL is a heretofore unappreciated feature of adult human mammary epithelia. Immunofluorescence staining for AXL expression in normal human breast tissue specimens detected infrequent AXL-expressing cells. A subset of the AXL-expressing cells in human mammary gland overlap with the K14/K19 double-positive population, a phenotype associated with multipotency (Shimono et al., 2009; Huo and Macara, 2014; Dravis et al., 2015; Lilja et al., 2018; Wuidart et al., 2018; Spike et al., 2012; Giraddi et al., 2018; Chen et al., 2017) (Figures 1A–1D). Small clusters of AXL+ epithelial cells co-expressing of luminal (K19) and basal (K14) markers were also detected in lobular acini (Figures 1E–1H). In ductal structures, AXL-expressing cells represent a unique population of cells with strong AXL staining (Figure 1I). RNA in situ hybridization (RNA-ISH) analysis of the ductal structures verified this expression pattern (Figure 1J (AXL, brown)). Epithelial cells with >15 AXL mRNA transcripts comprised a minority of cells of the mammary epithelium, consistent with a low Histo-score of 63 (Figure 1L). In contrast, RNA-ISH for the AXL-ligand GAS6 (Figure 1K), revealed a more widely distributed expression among most LEPs, and this heterogeneous expression pattern is correspondingly reflected in the higher Histo-score of 141 (Figure 1M). Dual RNA-ISH detected cells with GAS6 transcripts in LEP that were adjacent to the AXL-expressing cells in the same FFPE tissue sections (Figure 1N; RNA-ISH controls are shown in Figure S1). Collectively, these results suggested that AXL-expressing cells constitute a rare population of cells in the mammary epithelium. Some of the weaker staining AXL-positive cells in the ductal and lobular epithelium are K14+/K19+ positive, and based on RNA-ISH the GAS6 ligand is produced mainly by luminal cells.
Figure 1.
AXL Expression Is a Feature of Rare Adult Human Breast Epithelial Cells
AXL is expressed in rare epithelial cells in normal breast epithelium.
(A–D) (A) Multi-color immunofluorescence analysis of normal breast epithelium biopsies (n = 20). The ducts shown in (A–D) are formalin-fixed paraffin-embedded (FFPE) tissue sections stained with monoclonal antibodies against AXL (MAB10C9, white), MEP-specific cytokeratin 14 (K14, red), and luminal epithelial-specific cytokeratin 19 (K19, green); nuclear counterstain (DAPI, blue).
(E-H) Cryosections of normal human breast epithelium stained with monoclonal antibodies against: (E) AXL (ab21965, green), (F) K19 (magenta), (G) K14 (red). Overlay (H) reveals the areas of co-localized expression of AXL, K19, and K14 (white) in the lobular acini.
(I) Chromogenic IHC of AXL (ab21965, HRP-DAB brown) on FFPE sections of human mammary tissue. Counterstain by hematoxylin (blue).
(J–M) RNA in situ hybridization (RNA-ISH) on FFPE breast tissue specimens reveal the localization and distribution of (J) AXL mRNA transcripts, and (K) mRNA transcripts of the AXL-ligand GAS6. Each dot (brown, HRP-DAB) represents a single RNA molecule. (L and M) Histo-score analysis was performed to quantify the heterogeneity of the AXL and GAS6 mRNA transcripts within the breast epithelium at the single cell level. The Histo-score of transcript expression was determined by categorizing epithelial cells in five predefined bins based on the number of transcripts per cell. Distribution (% of cells/bin) as well as Histo-scores provided on a range of 0-400 is given.
(N) Dual RNA ISH on paraffin-embedded section of normal human breast specimen reveal the spatial distribution and juxtaposition of AXL (AP-based Fast Red) and GAS6 (HRP-based Green) mRNA transcripts within the normal human mammary epithelium. Counterstain by hematoxylin (blue).
The ducts shown in (A–D), (I), and (M–K), and (N), and the lobular acini shown in (E–H) are obtained from tumor biopsies from different patients.
Phenotypic Characterization of AXL-Expressing Cells in Human Breast Epithelium
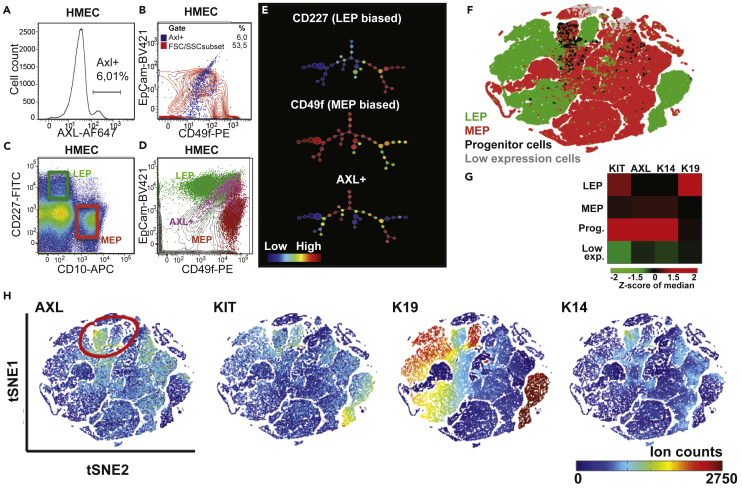
To further characterize the population of AXL+ cells in mammary epithelia, we analyzed primary uncultured human mammary epithelial cells (HMECs) from reduction mammoplasty specimens by flow cytometry using AXL antibody in addition to a panel of LEP, MEP, and stem/progenitor cell markers: CD227, CD10, EPCAM, CD49f. AXL-expressing HMEC represented 1–6% of the total HMEC population from different individuals (n = 5), which partitioned into an EPCAM+/CD49f+ subpopulation that is discrete from differentiated LEP (CD227+/CD10-) and MEP (CD227-/CD10+) populations (Figures 2A–2D). The EPCAM/CD49f/CD227/CD10/AXL FACS-analyzed HMECs were evaluated by spanning-tree progression analysis of density-normalized events (SPADE) to generate a putative hierarchical tree. The SPADE technique organizes groups of similar cells next to each other based on expression similarity, creating tree-like structures. AXL expression was highest in the node located at the apex of separate LEP (CD227 biased) and MEP (CD49f biased) radiations (Figure 2E). Next, we evaluated the expression of AXL, KIT, K14, and K19, among 25 other proteins, in a 29-marker mass cytometry (CyTOF) data set derived from dissociated primary breast epithelia of 57 women (Pelissier Vatter et al., 2018). Non-linear dimensionality reduction by t-distributed stochastic neighbor embedding projection and unsupervised clustering of intra-lineage subpopulations identified distinct LEP, MEP, and progenitor cells (Figure 2F). This high-dimensional analysis showed that AXL was primarily associated with KIT/K14/K19 expressing progenitor cells (Figures 2G and 2H). Notably, K14 was expressed relatively more than K19, which is anecdotally consistent with our immunofluorescence observations that K19 exhibits consistently lower, yet detectable, expression. Previously EpCAM+/CD49f + epithelia that express K14 and K19, and shared properties of LEP and MEP, were shown to be enriched for multipotent activity in human mammary epithelia (Villadsen et al., 2007). Epithelial cells expressing KIT were shown to be enriched for multipotent epithelial cell activity (Garbe et al., 2012; Lim et al., 2009; Pelissier et al., 2014). The finding that AXL also is expressed in epithelial cells bearing this same constellation of markers suggests that AXL may be a marker and regulator of multipotent cells in the human mammary epithelium.
Figure 2.
Phenotypic Characterization of AXL-Expressing Cells in Human Breast Epithelium
Analysis of AXL expression by flow cytometry of primary human breast epithelial cells (HMECs) isolated from patient reduction mammoplasty tissue samples.
(A) Total surface AXL staining of human breast epithelial cells isolated from epithelial-enriched preparations of reduction mammoplasty samples (range: 1–6%, n = 5 different patient biopsies; 500,000 events collected for each flow cytometry experiment displayed).
(B–E) (B) EPCAM/CD49f staining pattern of AXL-expressing cells (blue; gate shown in (E) within total human breast epithelial cell population (red topography map) (C) CD227/CD10 staining pattern and gating of LEP (green box) and MEP (red box) populations in epithelial-enriched preparations from reduction mammoplasty samples. (D) EPCAM/CD49f staining pattern and resolution of epithelial hierarchy cell types in enriched human breast epithelial and residual stromal cells isolated from reduction mammoplasty samples show enrichment of AXL-expressing cells in the stem/progenitor subpopulation (E) Analysis of HMEC EPCAM/CD49f/CD227/CD10/AXL flow cytometry data (from Figures 1C and 1D; 500,000 events; two different patients) using spanning-tree progression analysis of density-normalized events (SPADE), a computational approach to determine cell hierarchies from multiparametric data (Qiu et al., 2011). The SPADE-generated HMEC hierarchical tree comprises a continuum of distinct cell subpopulations depicted as circles with radii corresponding to cell number. The predicted HMEC hierarchy shows common origin at the apex with two radiations, MEP biased and LEP biased populations, respectively. Relative expression of a surface marker on cells within the hierarchy is shown on a blue (low expression) to red (high expression) scale. LEP-biased cells that express CD227, MEP-biased cells that express CD49f, and AXL-expressing cells are shown superimposed onto the HMEC hierarchy. Differentiated LEP and MEP cell populations occupy the left and right lineage radiations respectively, while AXL is expressed primarily in the putative bipotent epithelial stem/progenitor subpopulations found at the apex of the hierarchical tree.
(F) High-dimensional mass cytometry-based analysis of primary human mammary epithelia reveals a progenitor population expressing AXL, KIT K14 and K19. Non-linear dimensionality reduction, t-distributed stochastic neighbor embedding (tSNE) (Amir El et al., 2013) created a projection of 29 marker expression in 2D. Each point depicts a single cell of dissociated uncultured breast epithelia from women <30 years old (merged and subsampled at 50,000 cells, n = 7) (Pelissier Vatter et al., 2018). The raw data have been transformed with arcsinh with the cofactor of 5. Intra-lineage subpopulations were identified as distinct clusters of cells with shared phenotypes using PhenoGraph software (Levine et al., 2015). Unsupervised clustering identified four distinct phenotypes of LEP (LEP1–4), seven types of MEP (MEP1–7), a progenitor subpopulation and a low-expressing cell phenotype. The LEP and MEP clusters were merged and the tSNE projection of the PhenoGraph clusters is represented.
(G) Heatmaps of Z score of KIT, AXL, K14 and K19 expression in PhenoGraph clusters of uncultured breast epithelia from women <30 years old (merged, n = 7).
(H) tSNE projection (generated as described in detail for (F)), showing the relative expression of AXL, KIT, K19, and K14, respectively. The relative expression of these markers is shown on a violet-blue (low expression) to red (high expression) rainbow scale representing ion-counts from 0-27,500/cell.
KIT+/AXL + Primary HMECs Display a Unique Transcriptional Profile
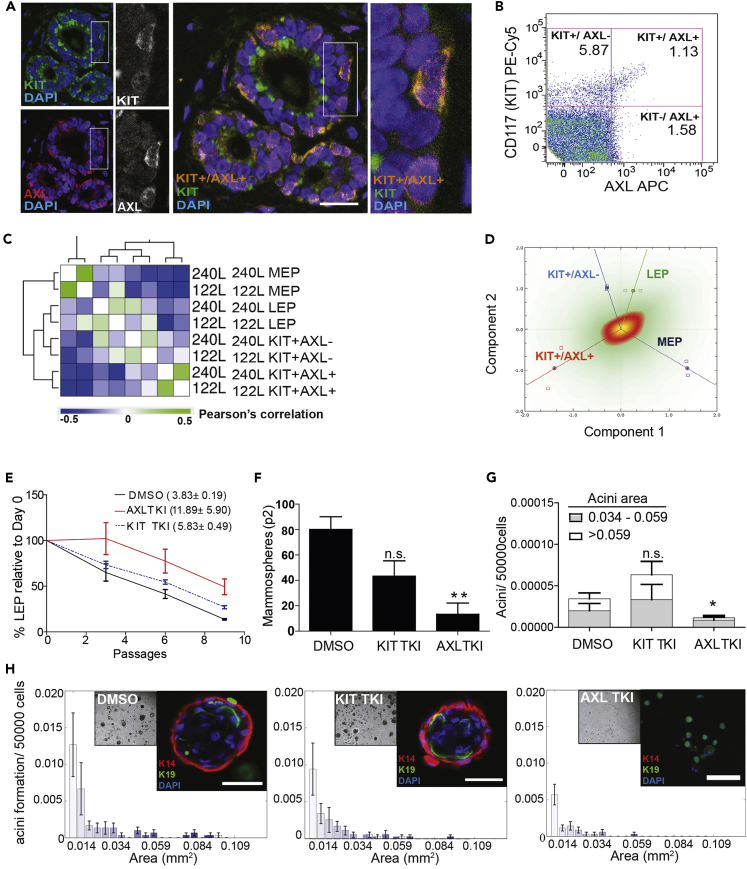
We next examined the distribution of AXL expression relative to KIT in situ. Immunofluorescence staining of normal breast tissue revealed that KIT+AXL+ breast epithelial cells were predominantly positioned basally relative to KIT+AXL− staining cells that were positioned adjacent to the lumen (Figure 3A). Primary HMEC were enriched by FACS based on expression of KIT, and AXL was found to be expressed by 16% of the KIT+ epithelial population, thus representing about 1% of the total primary mammary epithelial cell population (Figure 3B). To further delineate the difference between these epithelial populations, we sorted KIT+AXL+ and KIT+AXL− cells, and CD227+CD10- LEP and CD227−CD10+ MEP subpopulations from primary HMEC at fourth passage (from two different women) and performed whole-genome expression analysis. Unsupervised hierarchical clustering identified distinct KIT+AXL+, KIT+AXL−, LEP, and MEP populations (Figure 3C). Principal component analysis of gene expression data revealed strong separation of KIT+AXL+, LEP and MEP populations, whereas the KIT+AXL− HMEC showed greater similarity to differentiated LEPs (Figure 3D). These results are consistent with the hypothesis that KIT+AXL+ cells represent a distinct breast epithelial progenitor population compared to the KIT+AXL− population.
Figure 3.
AXL Is Required for Self-Renewal and Generation of Differentiated Acini from Human Breast Epithelial Cells Ex vivo
(A) AXL is co-expressed with KIT on breast epithelial cells. Immunofluorescence of normal breast epithelial ducts in normal human breast biopsy FFPE sections (n = 6) show AXL (red), KIT (green), and AXL+/KIT + double-positive cells (yellow). Nuclear counterstain by DAPI (blue). Scalebar: 30 μm.
(B) AXL expression defines a subpopulation of KIT-expressing breast epithelial progenitors. Flow cytometry-based quantification of KIT and AXL surface expression levels in human breast epithelial cells isolated from reduction mammoplasty samples (>100,000 sorted events; n = 3 patient samples). Quadrant gates and percentages of total events/gate are shown.
(C) AXL defines a distinct breast epithelial KIT-expressing progenitor subpopulation. Unsupervised hierarchical clustering based on whole-genome gene expression analysis (Illumina Bead Array) of FACS-isolated KIT+/AXL+, KIT+/AXL−, CD227+ luminal (LEP) and CD10 + myoepithelial (MEP) subpopulations of HMEC cells (independent FACS analysis of HMEC strains 240L and 122L at passage 4). Weighted average linkage (WPGMA); Distance metric: Pearson's Correlation.
(D) KIT+ progenitors lacking AXL show gene expression evidence of luminal commitment. Principal component correspondence plot derived from whole-genome gene expression analysis of KIT+/AXL+, KIT+/AXL−, CD227+ luminal (LEP), and CD10+ myoepithelial (MEP) FACS-isolated cells. X axis component variance: 22.387%; Y axis component variance: 17.091%; Total variance retained: 39.478%.
(E) Serial passage analysis of the percentage of CD227+/CD10- luminal cells (LEP) in the HMEC strains 240L and 122L seeded at passage 4 in the presence of DMSO (control), KIT tyrosine kinase inhibitor (TKI) (imatinib, 1 μM) or AXL TKI (bemcentinib, 600 nM). %LEP was normalized to passage 0. Observed half-life values + - SD for each treatment condition (insert) were calculated using the formula: t ∗ ln(2)/ln(N0/Nt) (p < 0.05).
(F) AXL activity is required for breast progenitor cell self-renewal as analyzed by secondary mammosphere formation generated by flow cytometry-enriched KIT + human mammary epithelial cell progenitors treated with DMSO (control), KIT TKI (imatinib, 1 μM), or AXL TKI (bemcentinib, 600 nM). Y axis represents total number of secondary mammospheres formed per well (mean ± S.E.M., n = 72; ∗∗p = 0.0075, t test).
(G) AXL is required for efficient formation of bilayered epithelial organoids in laminin-rich ECM assays. Image analysis of phase contrast images of mammary acini-like colonies formed from flow cytometry-enriched KIT + human mammary epithelial cell progenitor cells in 3D embedded laminin-rich ECM (lrECM) (matrigel), treated with DMSO (control), KIT TKI (imatinib, 1 μM), and AXL TKI (bemcentinib, 600 nM), respectively. Number of acini formed per 50.000 sorted KIT+ progenitor cells (mean ± S.E.M., n = 72; p values derived from t test) is shown by size distribution (Acini area: mm2).
(H) Size distribution of organoids formed under the conditions described i (G) (Area: mm2). Brightfield images and immunofluorescence analysis of representative organoids (inserts) display cytokeratin 14 (K14, red); cytokeratin 19 (K19, green); and nuclear counterstain: DAPI (blue). Scalebar 50 uM.
AXL Kinase Activity Is Required for Self-Renewal and Acini Formation in Primary HMECs Ex Vivo Assays
To address the function of AXL signaling during differentiation, we cultured HMEC in the presence of an AXL-specific small molecule tyrosine kinase inhibitor (TKI) and monitored changes in the percentage of LEPs during subsequent passages. Standard in vitro culture of HMEC does not maintain the LEP population effectively, with an observed half-life of 3.8 ± 0.2 days due to the favored expansion of cells with basal properties, such as MEP (Figure 3E) (Garbe et al., 2012). Addition of a selective AXL TKI (bemcentinib, 600 nM) (Holland et al., 2010) counteracted LEP loss in culture (observed LEP half-life of 11.9 ± 5.9 days; p = 0.43), consistent with a pro-luminal differentiation effect, whereas a KIT TKI (imatinib, 1 μM) did not significantly alter LEP half-life (5.8 ± 0.5 days) (Figure 3E). Next, we assessed the requirement of AXL signal transduction for in vitro secondary mammosphere formation by HMEC enriched for KIT expression through two passages. Secondary mammosphere formation assays are frequently used a measure of self-maintenance activity. Secondary mammosphere formation was not affected by treatment with imatinib, but was reduced more than 4-fold by treatment with the AXL-inhibitor bemcentinib (Figure 3F). Formation of multi-lineage acini in 3D laminin-rich ECM (lrECM) was also significantly inhibited by treatment with bemcentinib, and only a few single K19-staining cells were observed in culture post-treatment (Figures 3G and 3H). Functional ex vivo assays showed that AXL kinase activity is required for self-renewal and maintenance of multi-lineage differentiation potential of AXL+KIT+ HMECs. Taken together, in situ, multiparameter cytometry, gene expression, and functional cell-based ex vivo analyses of primary human mammary epithelia align and suggest that AXL+/KIT+ cells exhibit multipotent activity. AXL−/KIT+ cells reside in the luminal compartment and exhibit activity consistent with luminal-biased progenitors. Our data do not establish a direct hierarchy between the two cell types.
AXL Is Required for Regeneration of Mouse Mammary Glands upon Transplantation
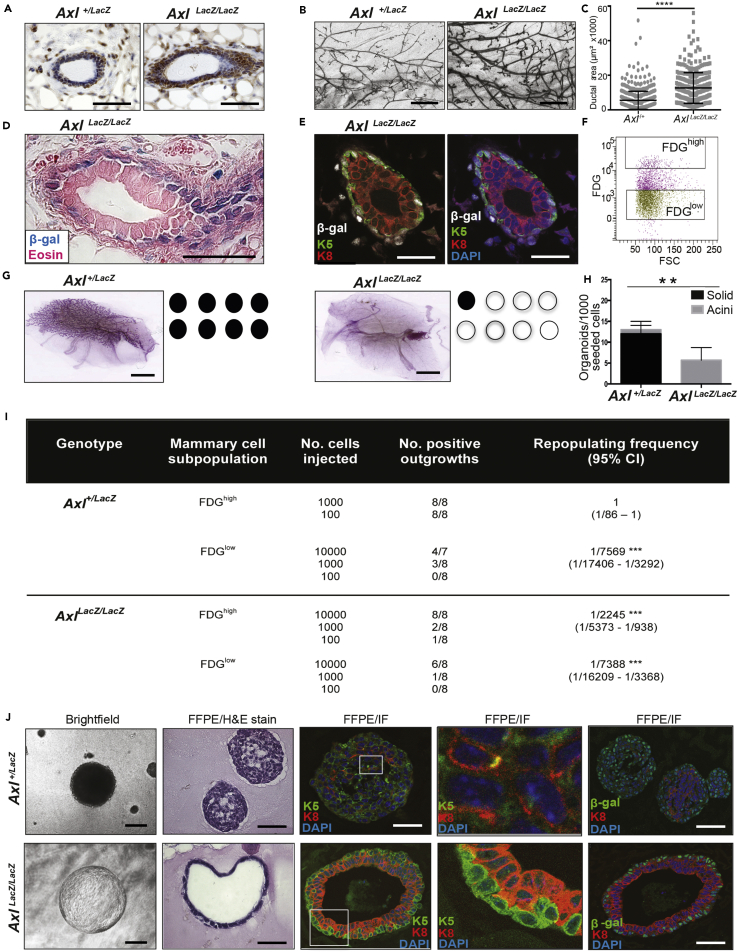
To explore the role of AXL in mouse mammary epithelia, we examined the mammary glands from a mouse strain that carries a targeted LacZ gene knock-in mutation that disrupts AXL protein expression (B6.129P2-Axltm1Dgen/J; Figures S2A and S2B). Analysis of the mammary glands of nulliparous adult expressing Axl+/+ (wild type) mice and the functional knockout AxlLacZ/LacZ mice showed no statistically significant difference in estrogen receptor alpha (ESR1), progesterone receptor, or proliferation marker Ki67 (Figures 4A, S3B, and S3D). These results indicated that differentiated lineages present in the luminal compartment of AxlLacZ/LacZ glands were similar to wild type (Figures S3B and S3D). Morphometric analysis of H&E stained FFPE tissue sections revealed a significant increase in the average caliber (cross-sectional area) of ducts from AxlLacZ/LacZ mice (n = 9) compared to ducts from wild type mice (n = 7, p < 0.0001) (Figure 4C). Image analysis of ductal diameters in carmine alum stained mammary gland whole mounts from 16-week-old AxlLacZ/LacZ (n = 9) and Axl+/+ wild type mice (n = 8) confirmed differences in the overall size distributions (Figures 4B and S3A). β-galactosidase histochemistry revealed basal localization of LacZ-expressing cells in adult ducts (Figure 4D), sites where mouse mammary multipotent epithelial stem cells are thought to reside (Joshi et al., 2012). Mammary ducts from AxlLacZ/LacZ glands stained by immunofluorescence for detection of transgene product Beta-galactosidase in combination with MEP-marker K5 and LEP marker K8 confirmed the basal localization of the cells harboring the LacZ transgene (Figure 4E).
Figure 4.
AXL Is Required for Regeneration of Mouse Mammary Glands upon Transplantation
(A) Immunostaining of FFPE sections of mammary glands from adult heterozygous Axl+/LacZ mice and homozygous functional AXL knockout AxlLacZ/LacZ of the B6.129P2-Axltm1Dgen/J mice revealed no significant difference in estrogen alpha receptor (ESR1) expression. Quantification is shown in Figure S3A. Scalebar: 100 μm.
(B) Carmine alum stained whole mounts of mammary glands from adult Axl+/LacZ and AxlLacZ/LacZ mice. Quantification of ductal diameter based on whole mounts are shown in Figure S3A. Scalebar: 250 μm.
(C) Morphometric histological analysis of HE stained mammary epithelial ducts from 16-week-old AXL+/+ (n = 7) and AxlLacZ/LacZ (n = 9) mice. Quantification of the epithelial ducts were evaluated from HE stained FFPE sections. Both inguinal glands were harvested and included in the analysis, and area of epithelial structures from 10 separate fields/gland were included in the analysis (Mann-Whitney p < 0.0001).
(D) AXL promoter drives β-galactosidase expression in the two AxlLacZ alleles of homozygous AxlLacZ/LacZ mice. Whole mount beta-galactosidase histochemistry reveal the transgene expression from Axl promoter activity of mammary epithelial ducts. Glands were FFPE embedded post-staining and sections counterstained by eosin. Scalebar: 50 μm.
(E) Immunofluorescent staining of β–galactosidase (white), K8 (red), and K5 (green) reveal the localization of transgene expressing cells relative to the luminal (K8+) and basal (K5+) population in mammary epithelial ducts of homozygous AxlLacZ/LacZ mice. Image without DAPI (blue) nuclear stain is displayed the right to allow better visual inspection of the transgene expression (white) relative to K8 (red) and K5 (green). Scalebar: 30 μm.
(F and G) (F) FACS sorted cells expressing high (5% upper gate) and low (65% lower gate) cells were used for subsequent in vitro assays and limiting dilution transplantation assays (G) Representative whole mount image of cleared mammary fat pad repopulated by 100 FDGhigh cells from heterozygous Axl+/LacZ mammary gland (left panel). Repopulating mammary epithelial outgrowths were found in 8/8 animals in this group (filled black circles). Representative whole mount image of mammary gland of animal reconstituted with 100 FDG+ cells from homozygous AxlLacZ/LacZ mammary glands (right panel). Repopulating mammary epithelial outgrowths were observed in 1/8 animals in this group (filled black, and empty circles, respectively). Data from one of three representative experiments are shown. Scalebar: 0.5 mm.
(H) Quantification of solid (black shaded bars) and acinar (gray shaded bars) in vitro colonies per 1000 seeded cells. Solid organoids were significantly reduced in the cultures derived from AxlLacZ/LacZ animals (t test: p = 0.0023).
(I) Limiting dilution in vivo mammary transplantation assay of FDGhigh and FDGlow cell populations. The number of mammary outgrowths per transplantation group were quantified 8 weeks post-implantation, and the stem cell frequency and confidence intervals were calculated by limiting dilution analysis using Extreme Limiting Dilution Analysis (ELDA) software (http://bioinf.wehi.edu.au/software/elda/) (Hu and Smyth, 2009).
(J) Images of of mammary epithelial cells in 3D embedded organoids derived from Axl-/LacZ cells (upper row) and AxlLacZ/LacZ cells in 3D laminin-rich ECM (lrECM) culture (lower row) reveal the undifferentiated solid organoids derived from Axl-/LacZ and the differentiated acini formed by AxlLacZ/LacZ cells. As indicated, images represent (from left to right) brightfield images of live organoid cultures; HE stained organoids in formalin-fixed paraffin embedded (FFPE) sections, and immunofluorescence (IF) of FFPE sections with the markers K5 (green), K8 (red) and K5/K8 co-staining (yellow). The pictures to the right in the panel show beta-gal (green), K8 (red). Counterstain by DAPI (blue). Scalebars: 50 μm.
The observed phenotype of Axl LacZ/LacZ mammary glands is consistent with the viability of Axl-knockout animals; however, we speculated that forced postnatal regeneration of the mammary epithelia would exert sufficient stress to overcome compensatory mechanisms and reveal a more penetrant Axl-null phenotype. Thus, we evaluated the effect of AXL on mammary gland reconstitution upon transplantation of sorted cells in serial dilution. Lineage-negative (i.e. CD45-, CD31-, CD11b−) AXL-expressing cells were enriched from dissociated Axl+/LacZ and AxlLacZ/LacZ adult mammary glands by FACS using the fluorogenic β-galactosidase substrate fluorescein Di-β-D-galactopyranoside (FDG). An FDGhigh gate (highest 5%) was used to enrich for AXL-expressing cells, while AXL-negative cells were sorted using an FDGlow gate (lowest 65%) (Figure 4F). Serial dilutions of cells sorted on the basis of FDG (from 10,000 to 100 cells) were implanted into cleared fat pads of recipient prepubescent nude mice. Eight weeks post-transplantation, the mammary glands were harvested, fixated, and whole mount stained with carmine alum to visualize epithelial outgrowths. Mammary repopulating units (MRUs) frequencies were estimated by extreme limiting dilution analysis. FDGhigh Axl+/LacZ cells readily repopulated the cleared fat pads with extensive mammary epithelial trees, whereas FDGlow Axl+/LacZ cells displayed a significantly reduced MRU frequency, as did the AxlLacZ/LacZ cells (Figures 4G and 4I). Hence, sorting for AXL expression enriched for adult murine mammary cells with repopulating activity in ex vivo organ reconstitution assay.
A number of previous reports show that luminal-biased progenitor cells cultured in 3D lrECM form hollow acini, whereas isolated MRU-competent MaSC form dense, pleomorphic structures (Lim et al., 2009; Shackleton et al., 2006; Stingl et al., 2006; Guo et al., 2012). FACS-isolated FDGhigh mammary epithelial cells from Axl+/LacZ mice formed solid basal/stem-like colonies in lrECM characteristic of regenerative MaSC (Figures 4H and 4J upper row), whereas the FACS-isolated FDGhigh mammary epithelial cells from AxlLacZ/LacZ mice formed significantly less colonies, and the colonies were predominantly well differentiated bilayered acini with basal K5 expressing and luminal K8 expressing compartments of cells (Figures 4H and 4J lower row).
Transcriptome of Sorted AXL + Mammary Cells Are Enriched in Genes Characteristic of Sorted MaSC Populations
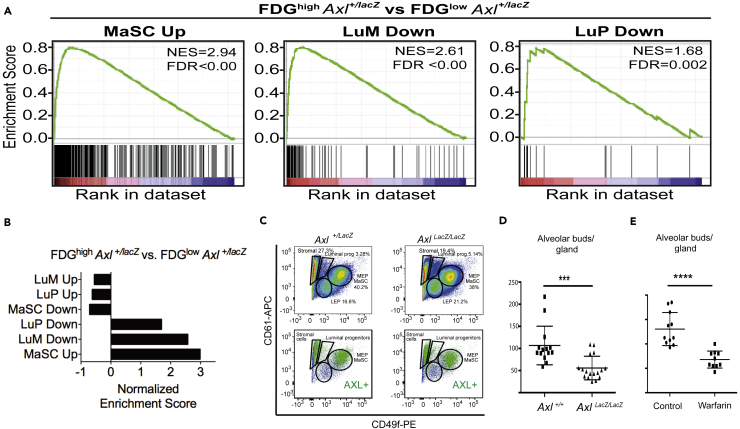
Transcriptomes from FACS sorted FDGhigh and FDGlow cells isolated from Axl+/LacZ adult mammary glands were then compared to previously published gene expression signatures from sorted mammary epithelial populations (Lim et al., 2009). Consistent with the functional analyses described above, the sorted FDGhigh cell population isolated from Axl+/LacZ mammary glands displayed significant transcriptional similarity with MaSC populations enriched using previously described markers (CD49fhi CD29hi CD24+ Sca1-subset) (Figures 5A and 5B). Indeed, CD61/CD49f flow cytometry analysis of dissociated mammary glands showed an increase in the luminal progenitor and LEP populations, and a slight reduction of the basal/stem population in cells from AxlLacZ/LacZ mice compared to Axl+/LacZ mice (Figure 5C). Whole mount adult mammary gland epithelia from AxlLacZ/LacZ mammary glands displayed a significant reduction in the number of alveolar buds at ductal termini compared to Axl+/+ mammary glands (Figure 5D).
Figure 5.
Gene Signatures Associated with Mammary Stem Cells (MaSC Population) Are Enriched in Murine Mammary Gland AXL + Cells and Loss of AXL Promotes Differentiation
(A) The gene set enrichment analysis of FDGhigh versus FDGlow populations of Axl-/LacZ mammary cells revealed high similarity with published gene expression datasets from the MaSC-population enriched using standard markers (Lim et al., 2009). Enrichment plots for: MaSC = mammary stem cells; LuM = Luminal mature; LuP = Luminal progenitors, are shown. The top portion of the plot shows the running enrichment score for the gene set (green line) as the analysis moves along the ranked list of genes (x axis; red color indicates genes with higher expression and blue indicates low expression when the two gene-sets are compared). NES = normalized Enrichment Score. FDR = False discovery rate.
(B) The gene set enrichment analysis of FDGhigh versus FDGlow populations of Axl-/LacZ mammary cells. NES = Normalized Enrichment Score is given for the comparison with the previously described gene signatures of MaSC = mammary stem cells; LuM = Luminal mature; LuP = Luminal progenitors (D) The gene set enrichment analysis of FDGhigh versus FDGlow populations of AxlLacZ/LacZ mammary cells.
(C) Expression of CD61 and CD49f in lineage-negative cells isolated from Axl-/LacZ (left) and AxlLacZ/LacZ (right) mammary glands. Gating and percentage of total cells for different mammary cell populations is shown. Back gating of FDGhigh cells confirm that AXL expression more prominent in the CD49fhi/CD61+ MEP/MaSC population than in the CD49flow/CD61low LEP population.
(D) Quantification of alveolar buds at the termini of ducts of mammary glands were performed on whole mounts from Axl+/+ (wild type, n = 8) and AxlLacZ/LacZ (AXL-null, n = 8) mice, 2 inguinal glands per animal (t test, p < 0.0001).
(E) Quantification of alveolar buds at the termini of ducts of mammary glands from mice treated for 5 months with warfarin (1 mg/L in drinking water). Quantification were performed on inguinal mammary gland whole mounts (n = 10 control glands, n = 10 warfarin treated glands), p=<0.0001, t test.
We hypothesized that AXL regulation of MaSC luminal potency in adult mammary gland regeneration could be governed by juxtacrine interactions between GAS6-producing LEP and AXL-expressing MaSC. Warfarin has previously been shown to be a well-tolerated inhibitor of GAS6-AXL signaling (Kirane et al., 2015). Thus, to test this model, we treated adult mice (12 weeks old) for 5 months with warfarin administered ad libitum in the drinking water to inhibit post-translational glutamic acid gamma-carboxylation of GAS6. Gamma-carboxylation of GAS6 has been shown to be required for strong AXL activation, and warfarin, a gamma-carboxylation inhibitor, converts GAS6 into a selective AXL antagonist (Lew et al., 2014; Kirane et al., 2015). Analysis of whole mounted adult mammary glands after 5 months of warfarin treatment demonstrated a significant reduction in the number of terminal alveolar buds in the warfarin treated group (Figure 5E), which phenocopied the AxlLacZ/LacZ animals (Figure 5D). A significant reduction in the number of terminal alveolar buds/gland was furthermore detected in pubescent warfarin treated mice (Figures S4A–S4C). Collectively, these results are consistent with a pro-differentiation phenotype of mammary glands of nulliparous adult Axl-null animals, as well as in AXL-antagonized wild type animals.
AXL MaSC Regulation Is Associated with Breast Cancer
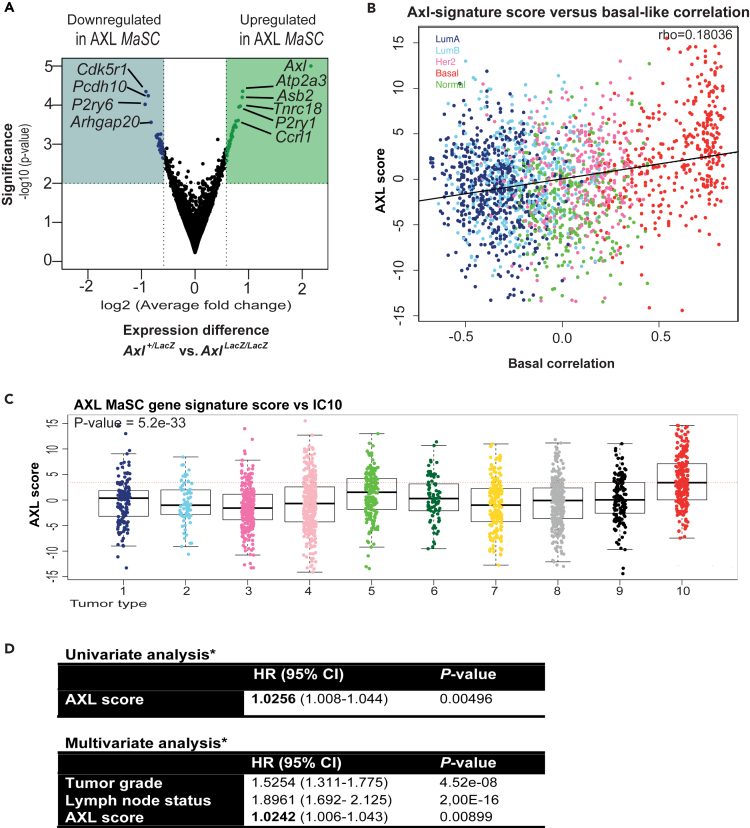
We next examined whether gene signatures of normal AXL+ MaSC were an underlying feature of mammary tumors. Gene set enrichment analysis was used to detect the most differentially expressed genes between sorted FDGhigh Axl+/LacZ and AxlLacZ/LacZ mammary cells. A signature comprising 33 downregulated and 37 upregulated genes was identified (rank product test, Table S1). Genes in this set have not been previously associated with MaSC, and thus may represent an unprecedented facet of the regenerative adult MaSC cell state (Figure 6A and Table S1). Notably, the AXL MaSC gene signature (AXL-stem) is not significantly enriched for core EMT genes. The generic EMT score captures the universal features of EMT based on expression of 315 EMT-related genes, and computed EMT scores range from −1 (epithelial) to 1 (mesenchymal) (Tan et al., 2014). The FDG high sorted cell population of Axl+/LacZ mice has a mean generic EMT score of 0.24, while FDG high sorted cells of AxlLacZ/LacZ mice have a mean generic EMT score of 0.21. Thus, these comparable intermediate mesenchymal EMT scores support a heretofore unappreciated role for AXL signaling in epithelial plasticity more generally, rather than maintaining EMT per se.
Figure 6.
AXL MaSC Regulation Is Associated with Breast Cancer
(A) Volcano plot of significance versus fold gene expression change between the FDGhigh populations from Axl+/LacZ and AxlLacZ/LacZ adult mouse mammary epithelial cells. The most highly differentially expressed genes (cutoff: 1.5-fold change) are shaded.
(B) The Metabric breast cancer patient cohort (n = 1,980 patients) (Curtis et al., 2012) was interrogated with the AXL MaSC gene signature (AXL-stem, Table S1) to access the distribution correlated with the degree of basal-like gene expression in breast cancer (ρ = 0.181, p = 5.5 × 10-16).
(C) The Metabric breast cancer patient cohort (n = 1,980) (Curtis et al., 2012) was interrogated with the AXL MaSC gene expression signature (AXL-stem, Table S1) to assess the influence of the AXL stem gene expression signature on clinical endpoints. AXL-stem score was significantly elevated in the core basal subtype of integrative cluster 10 (IC10) subtyped tumors from the Metabric breast cancer patient cohort (n = 1,980) (Curtis et al., 2012) (p = 5.2 × 10-33, Kruskal-Wallis rank test).
(D) AXL-stem score was associated with breast cancer-specific outcome in a univariate model, stratified for hospital (p = 0.00496; hazard ratio: 1.026) and in a multivariate model correcting for grade and lymph node status (p = 0.00899; hazard ratio: 1.024, Cox proportional hazards regression, Breitling et al., 2004).
We and others have previously showed that AXL protein and mRNA expression is frequently detectable in basal-like breast cancer subtypes (Gjerdrum et al., 2010; Blick et al., 2010). The AXL-stem signature correlated with the degree of basal-like gene expression in the Metabric breast cancer patient cohort (Curtis et al., 2012) (Figure 6B) and was significantly elevated in basal-like breast tumor subtypes (PAM50 and IC10 subtypes; Figures 6C and S5). Furthermore, the AXL-stem score was significantly associated with breast cancer-specific patient survival in a univariate model and in a multivariate model correcting for grade and lymph node status (Figure 6D). Hence, the AXL-dependent gene expression signature from murine MaSC correlates with clinical outcome in human breast cancer.
AXL Signaling Is Required for Breast Cancer Cell Phenotypic Plasticity
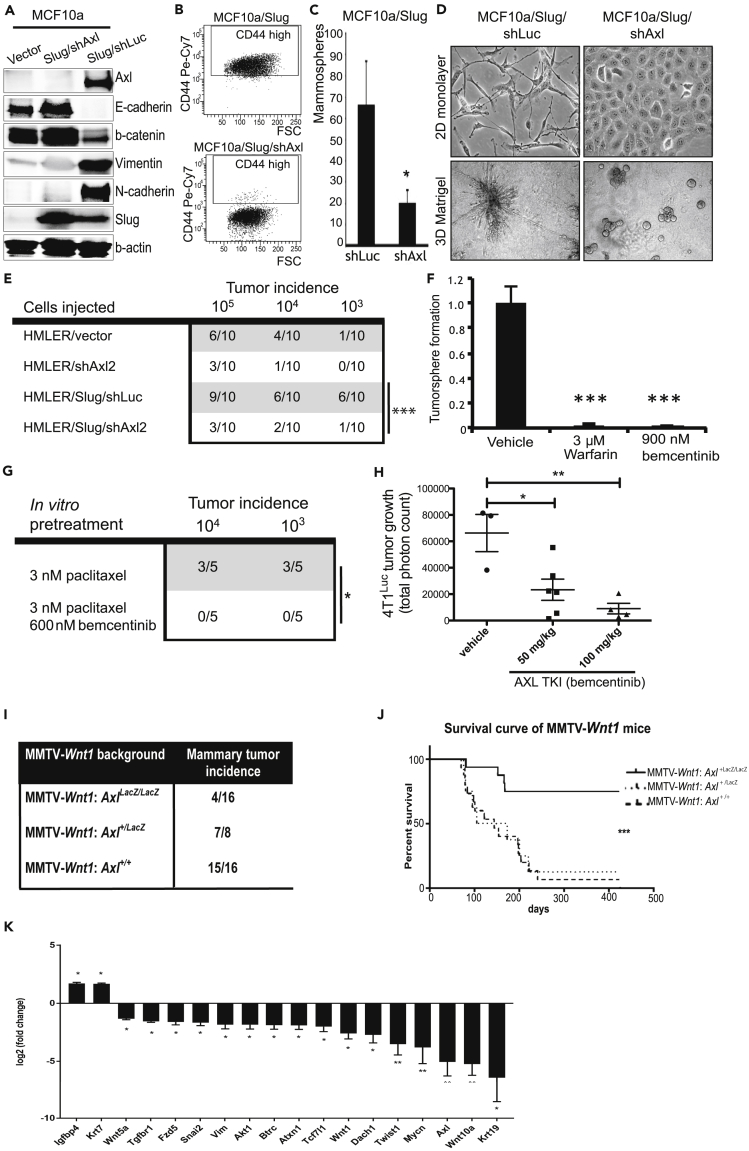
Expression of the AXL-stem genes in breast cancer may reflect a co-option of MaSC-related regenerative activity that drives epithelial plasticity in breast cancer. SNAI2/Slug expression in immortalized human breast epithelial cell lines such as MCF10A engages an EMT-related stem cell program that results in expression of the AXL RTK (Gjerdrum et al., 2010; Vuoriluoto et al., 2011; Jokela et al., 2018). AXL knockdown were shown to reverse the SNAI2/Slug-dependent mesenchymal phenotype, restoring epithelial morphology and molecular marker expression, and blocking sphere-forming activity, without affecting SNAI2/Slug protein levels (Figures 7A–7D). Ectopic expression of AXL did not drive EMT in MCF10a (Figure S6). These results indicated that AXL signaling is required for SNAI2/Slug-dependent regulation of the EMT program. HMLER is a cell line generated by oncogenic transformation of HMECs that displays increased tumorgenicity upon overexpression of SNAI2/Slug (Mani et al., 2008). AXL knockdown significantly decreased tumor incidence independent of SNAI2/Slug overexpression in this model (Figure 7E). The 4T1 murine mammary tumor model enables tumor-forming experiments in isogenic, immune-competent mice. Tumorsphere formation with 4T1 cells was nearly abolished by treatment with warfarin (3uM) or with bemcentinib (900 nM) (Figure 7F). Pretreatment of 4TL with paclitaxel (3 nM) reduced tumor incidence following implantation, and pretreatment with paclitaxel and bemcentinib (600 nM) completely prevented tumor formation (Figure 7G). Furthermore, bemcentinib administered in vivo (both 50 and 100 mg/kg) decreased 4T1 tumor growth in syngeneic host mice (Figure 7H). These results support the notion that AXL is required to sustain epithelial plasticity traits that facilitate tumor formation in mammary glands.
Figure 7.
AXL Is Required for Breast Cancer Cell Phenotypic Plasticity, and Genetic Ablation of AXL Significantly Reduces Mammary Tumor Incidence in the MMTV-Wnt1 Mice
(A) AXL expression is required for SNAI2/Slug-induced EMT. MCF10a cells transduced with control or SNAI2/Slug retroviral expression vectors (GFP), and AXL-targeting shRNA (shAXL) or luciferase targeting control shRNA vectors (shLuc) (RFP). Transduced cells were sorted based on GFP and RFP expression, and analyzed for AXL (140 kDa), SNAI2/Slug (30 kDa), and epithelial markers E-cadherin (135 kDa), β-catenin (92 kDa) and mesenchymal markers vimentin (55 kDa), and N-cadherin (100 kDa) by Western blotting. Loading control: β-actin (42 kDa).
(B) AXL-dependent loss of cell-surface glycoprotein CD44 shown by flow cytometric analysis of MCF10a/Slug/shAXL and MCF10a/Slug/shLuc cells.
(C) AXL expression is required for self-renewal activity. Quantification of mammosphere formation by MCF10a/Slug/shAXL and MCF10a/Slug/shLuc cells. Y axis represents total number of mammospheres formed per well (mean ± S.D., n = 5; ∗p < 0.05, t test).
(D) Phase contrast images of MCF10a/Slug/shAXL and MCF10a/Slug/shLuc cells grown as monolayer (2D, upper) and colony formation in 3D embedded laminin-rich ECM (lrECM) (matrigel, lower) reveal that Slug-mediated mesenchymal cell morphology and invasiveness are AXL-dependent.
(E) AXL is required for tumor initiation in vivo. Tumor incidence of HMLER/shLuc, HMLER/shAXL2 and HMLER/Slug/shAXL2 and HMLER/Slug/shLuc cells injected s.c. into recipient NOD-SCID mice at limiting dilutions (between 106-103 cells). HMLER/Slug/shAXL2 versus HMLER/Slug/shLuc, p = 0.0002, Fisher's exact test.
(F) Inhibition of AXL signaling using warfarin (3 uM) or AXL tyrosine kinase inhibitor (TKI) bemcentinib (900 nM) blocks 4T1 tumorsphere formation. Tumorsphere formation (day 7) was scored as Total Area (pixels2)/20,000 cells using ImageJ Analysis. Data plotted relative to vehicle (DMSO) treated cells (mean ± S.D., n = 6; ∗∗∗p = 0.0005, t test).
(G) Reduced tumor incidence of 4T1 cells pretreated in vitro with 3 nM paclitaxel in the presence of 600 nM bemcentinib prior to injection injected into syngeneic host mice at limiting dilution versus in vitro treatment with 3 nM paclitaxel alone (p = 0.0108, Fisher's exact test).
(H) Inhibition of AXL kinase activity reduces mammary tumor formation. Bioluminescence (total photon counts) from tumors formed from orthotopically-implanted 4T1-luciferase (4T1Luc) cells at Day 7 treated with bemcentinib (50 and 100 mg/kg QD) (mean ± S.D., n = 6; ∗p < 0.05, ∗∗p < 0.005, one-way ANOVA).
(I) Spontaneous mammary tumor incidence in genetically modified animals carrying MMTV-Wnt1 and the AXLLacZ knock-in allele. Comparison of tumor incidence between female AXL wild type MMTV-Wnt1:Axl+/+ and AXL wild type MMTV-Wnt1:Axl+/LacZ and AXL-null MMTV-Wnt1:AxlLacZ/LacZ animals revealed that Wnt1-induced mammary tumor incidence (within 14 months) was significantly reduced in the AXL-null background (p = 0.0002, Fisher's exact test, two-tailed).
(J) Kaplan-Meier survival analysis of MMTV-Wnt1 animals (Log rank (Mantel-Cox) test, Chi square: 14.98, p = 0.0001).
(K) Relative mRNA expression levels of a selection of significantly deregulated genes associated with EMT, CSC and WNT pathways in tumor cells isolated from MMTV-Wnt1:AxlLacZ/LacZ (n = 4) versus MMTV-Wnt1:Axl+/+ (n = 6) animals. The expression levels of the MMTV-Wnt1:AxlLacZ/LacZ tumors were quantified relative to MMTV-Wnt1:Axl+/+. Data are reported as mean fold changes ±SEM after normalization to the levels of housekeeping genes (ACTB, B2M, GAPDH, GUSB, HSP90AB1) in the panel. ∗p < 0.05, ∗∗p < 0.01 (Unpaired Student's t test).
AXL Is Required for Efficient MMTV-Wnt1 Mammary Tumorigenesis
Mammary tumors arise in the MMTV-Wnt1 model within an expanded MaSC pool and aberrant multipotent progenitor cells (Lim et al., 2009; Vaillant et al., 2008). Finally, in order to investigate the role of AXL in Wnt1-induced malignant transformation, we crossed the AxlLacZ knock-in and MMTV-Wnt1 mouse strains. As expected, female MMTV-Wnt1:Axl+/+ and MMTV-Wnt1:Axl+/LacZ animals developed mammary tumors with high penetrance (Figure 7I). Strikingly, the incidence of mammary tumor development was reduced in the functional Axl-knockout MMTV-Wnt1:AxlLacZ/LacZ animals (Figure 7I), reflected also by the significantly increased survival of these animals (Figure 7J). Thus, AXL expression supported efficient Wnt1-mediated tumorigenesis, and comparison of gene expression in mammary tumor cells isolated from MMTV-Wnt1 animals revealed significantly reduced expression of WNT, EMT, and cancer stem cell genes in rare tumors from the Axl LacZ/LacZ background (Figures 7K and S8).
Discussion
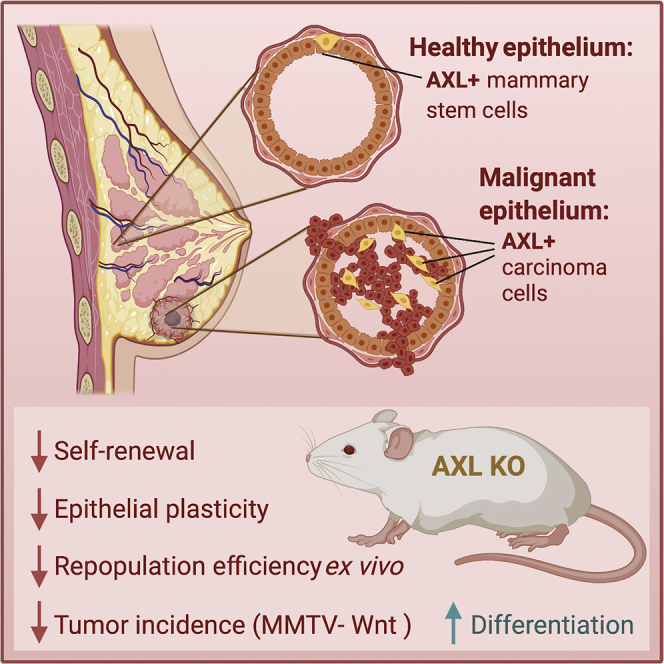
AXL is expressed in multiple cancer types and is associated with poor clinical outcome and resistance to a number of therapies, including immune checkpoint inhibitors (Davidsen et al., 2017; Hugo et al., 2016; Goyette et al., 2018). While the presence of AXL in pathological contexts is increasingly well documented, our understanding of the role of AXL in normal physiology is lacking, with the current study providing evidence of a conserved role for AXL in human and murine mammary gland outside of innate immune regulation.
Supported by analysis of healthy human breast tissue, genetic mouse models, and patient tumor gene expression, our results are consistent with the interpretation that AXL is expressed by epithelial cells that are in a stem cell state. Our data suggest that AXL is so far a singular example of a putative MaSC marker that is conserved in human and mouse mammary epithelia. Data support the role of AXL as a regulator of a cellular plasticity program that endows a rare proportion of HMECs with the ability to transition between multipotent and differentiated states. Our multiparametric gene and marker expression data sets as well as functional ex vivo analyses are consistent with the interpretation that AXL is required for conditional expansion of a multipotent MaSC population in the adult mammary gland of both humans and rodents. In particular, AXL was found to regulate a unique gene set, AXL-stem, in regenerative MaSC that was inclusive of ARTN, is a GDNF-family/syndecan-3 ligand, a gene family associated with stem cell self-renewal (Merrell and Stanger, 2016), and linked to EMT and drug resistance traits in breast cancer (Kang et al., 2009; Ding et al., 2014). The AXL-stem signature identified in murine MaSC correlated with clinical outcome in the human Metabric breast cancer patient cohort. Significantly elevated in basal-like breast tumor subtypes (PAM50 and IC10 subtypes), the AXL-stem signature was correlated with poorer overall survival across all breast cancer subtypes. AXL may allow breast cancer cells the capacity to transition between distinct phenotypic states with different functional traits that support tumorigenesis (Visvader and Stingl, 2014). More work is necessary to determine if AXL signaling is a characteristic of long-lived multipotent MaSC or whether it is necessary for expansion and acquired multi-lineage differentiation potential during certain regenerative conditions (Rios et al., 2014; Van Keymeulen et al., 2011).
AXL was shown to be required for the efficient formation of bilayered acini in lrECM assays from FACS enriched KIT + HMECs, and AXL inhibition prevented LEP loss in 2D HMEC culture and significantly decreased self-renewal capacity in secondary mammosphere assays. Although we cannot rule out the possibility that AXL+ MaSC are luminal-biased, both mammary transplantation in cleared murine fat pads and acinus formation assays with primary human cells showed a capacity for self-renewal and multi-lineage differentiation at least ex vivo. Multi-color immunofluorescence staining of mammary tissues showed that AXL + cells expressed also the intermediate filament proteins K14 and K19 that were located in a basal or suprabasal position, in ducts. FACS staining showed that AXL + cells were enriched among EpCAM+/CD49f+ cells. Our previous work showed that EpCAM+/CD49f+ HMECs that exhibited stem cell activity co-expressed K14 and K19 (Villadsen et al., 2007). EpCAMlow/CD10high epithelial cells also were enriched for stem cell activities and were reported to express K14 and K18, but not K19 (Bachelard-Cascales et al., 2010). Taken together, we interpreted these findings such that cells in MaSC states have shared properties of MEP and luminal cells. From the human high-dimensional expression data, it is evident that the KIT+AXL− HMEC are more luminal lineage-biased, and their localization adjacent to the lumen, and the similarity of gene expression to CD227+/CD10-/KIT− luminal cells supported this interpretation. However, more work and lineage tracing experiments are needed to determine definitively the lineage potential of the AXL + mammary epithelial population.
A conundrum raised by our results is that Axl-null animals are able to develop functional mammary glands, yet upon ex vivo transplantation into cleared fat pads of recipient mice the sorted Axl-null epithelial cells showed a significant reduction in the ability to reconstitute the mammary tree. Relatedly, the GAS6-null mouse also develops functional mammary glands (Mills et al., 2018). Knockout of the TAM family member Mer did show a developmental phenotype during post-lactational involution as it was shown necessary for mediating epithelial efferocytosis of apoptotic cells, but AXL and Tyro3 were explicitly not required for that process (Sandahl et al., 2010). The importance of the mammary gland for mammals implies strong evolutionary pressure to favor gland development with much redundancy. Indeed, we are not aware of any reports of gene knockouts that reach adulthood and have no mammary gland. AXL is an imprinted gene, which rather suggests that fine-tuned temporal and spatial regulation of AXL expression during embryonic development is crucial. AXL + epithelial cells were enriched for their ability to form mammary glands in the cleared fat pad assay, which may be a better representation of regeneration or even wound repair. Historically, a number of gene knockout animals have shown no overt phenotype until stressors were applied, and this may be an example in which AXL was essential for allowing the mammary epithelial cells to survive in, or even to establish, the regenerative niche. Furthermore, as we have noted above, the role of AXL in cancer progression is being increasingly appreciated and the regenerative or wound healing microenvironment has also been used to characterize the tumor microenvironment. Even though cell transfer and tissue regeneration assays are considered a gold standard for stem cell biology, it is worth recognizing that the assays are prone to disrupting solid tissues in a way that might be orthogonal to development.
A reasonable interpretation of our data is that AXL allows access to gene expression programs that repress the luminal phenotype. Hence, removal of signaling through the AXL pathway is sufficient to allow cells to access the luminal differentiation program. Indeed lineage-priming occurs in the expanded stem cell population during pregnancy prior to commitment along the alveolar lineage (Pal et al., 2013). Interfering with AXL expression in double-positive epithelial cells may push the progenitor profile into a different compartment, or the cells lose self-renewal abilities in favor of terminal differentiation due to a transition from a symmetric to an asymmetric mode of stem cell division. Although the mode of stem cell division was not directly addressed in this study, the increased mammary tumor incidence in the MMTV-Wnt model as well as the impact of AXL inhibition in ex vivo assays could support a model where AXL act as a mediator of symmetric self-renewing stem cell division, and the differentiation induced upon AXL inhibition is due to a switch to an asymmetric or symmetric differentiating stem cell division which is less advantageous for stem cell expansion and neoplastic transformation.
The striking reduction of Wnt1-driven mammary tumors in the Axl-null background was associated with a reduced expression of WNT-signaling genes (e.g. Wnt5a, Wnt10a, Wnt1, Fzd5, Mycn), genes associated with EMT (e.g. Twist1, Snai2, Vim), and K19 and Tgfb1/Tgfbr1 expression. It is tempting to speculate that AXL may support Wnt1-induced multipotent stem-like properties (Vaillant et al., 2008). Further characterization of the MMTV-Wnt1 tumor data is required; more mechanistic insights should be gathered to determine if Axl depletion leads to an impairment of Wnt signaling, or whether the effect is independent of Wnt.
We recently found that warfarin-use is associated with lower cancer risk in a large retrospective epidemiology study (Haaland et al., 2017). The vitamin K antagonist warfarin selectively inhibits gamma-carboxylation of Gla domain proteins, of which there are only 14 encoded in the human genome, most representing coagulation factors predominantly expressed in the liver. GAS6 is an outlier in this group with expression in several tissues and whose only known function is as the sole ligand for AXL (and a shared lower affinity ligand for MERTK/TYRO3). Of note, GAS6 is an estrogen inducible gene in mammary epithelial cells, and by bridging the kinase ectodomain and externalized phosphatidylserine (PS) displayed e.g on apoptotic cells, gamma-carboxylation of the GAS6 Gla domain is required for strong AXL activation, but not for MERTK/TYRO3 activation (Lew et al., 2014). Thus, “Gla-less” GAS6 is a specific AXL antagonist (Lew et al., 2014). Relevant to our warfarin experiments, and unaccounted for so far, is the possibility that other Gla domain proteins (periostin and osteocalcin) that are mainly expressed in the stroma were affected in a way that alters mammary epithelial activity.
Based on the data presented here we propose a working model where juxtracrine GAS6 and AXL interactions support microenvironmental PS-sensing and adult mammary gland stem cell expansion in the context of tissue damage and regeneration that deserves to be further explored in relevant models. Collectively, the results support further exploring aberrant AXL signaling as a therapeutic target in the treatment as well as chemoprevention of breast cancers.
Limitations of the Study
The study of human breast epithelial stem cells is hampered by a lack of experimental models and thus our current understanding of mammary gland biology is derived largely from rodent models. Although a number of flow cytometric markers are used to enrich for MaSC, a consensus set of markers that identifies multipotent MaSC in both human and mouse mammary remain elusive. This lack of common markers and mechanistic understanding governing human and mouse multipotent MaSC has brought into question how congruent MaSC biology is between these two species, casting doubt on the relevance of results determined in mouse studies, particularly for breast cancer. Furthermore, it remains to be elucidated whether regeneration of adult mammary tissue from endogenous stem cells exploits the same molecular pathways used to establish the mammary epithelium during development. As the ability of sorted cells to form mammary glands in the cleared fat pad assay may be a better representation of regeneration, and the role of GAS6-AXL signaling in murine mammary gland development have not been addressed. From emerging high-dimensional data, one can speculate that a continuum of cells along the differentiation axis is far more complex than previously anticipated and remains to be explored further.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, James Lorens (jim.lorens@uib.no).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact upon request pending completed Materials Transfer Agreement.
Data and Code Availability
The CyTOF data reported in this paper is accessible through Mendeley: https://doi.org/10.17632/j7mrbgt3hh.1. The BeadChip array data of sorted cells from B6.129P2-Axltm1Dgen/J mice are accessible through GEO database, accession number: GSE156662: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE156662.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors are grateful to Dr. Janice Nigro for invaluable advice, language and manuscript editing. Thanks to Sissel Vik Berge, Endre Stigen, Ingrid Gavlen, Bendik Nordanger, and Jason Toombs for excellent technical support, Brith Bergum and Marianne Enger at the Flow Cytometry Core Facility at UiB, and Endy Spriet and Hege Avsnes Dale at the Molecular Imaging Center, UiB. The authors thank Dr. Kjell Petersen, Computational Biology Unit and, Norwegian Bioinformatics Platform, UiB, for microarray analysis support. The authors are grateful to the staff at the animal facility at the University of Bergen for their skillful maintenance and care of the research animals. And finally, the authors express their thanks to the women that consented to the use of their reduction mammoplasty tissue and biopsy tissue for this research.
This work was partly supported by the Research Council of Norway through its Centers of Excellence funding scheme, project number 223250 (CCBIO affiliates). JBL was supported by grants from the Norwegian Research Council (grant number 204868), and Norwegian Cancer Society (grant number 190330). ML from the National Institutes of Health (R01AG040081 and R00AG033176), and United States Congressionally Directed Medical Research Program's Era of Hope Scholar Award (BC141351). ASTE was supported by an FRIPRO Mobility Grant Fellowship from the Research Council of Norway co-funded by the EU's seventh Framework Program's Marie Skłodowska Curie Actions (MSCA COFUND, grant agreement number 608695). Support by Legat for Forskning av Kreftsykdommer fund at UIB, and Familien Blix fund to ASTE for the conduct of this study is greatly appreciated. Graphical abstract was created with BioRender.com.
Author Contributions
Conception and design: M.A.L, J.B.L. Development of methodology: A.S.T.E, K.W-L, S.B, F.A.P.V, R.V, C.T, M.M, S.G., M.R.S, M.A.L, J.B.L. Acquisition of data: (provided animals, acquired and managed patients, provided facilities, etc.): A.S.T.E, K.W-L, S.B, F.A.P.V, C.T, M.M, O.S. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A.S.T.E, K. W-L, S.B, F.A.P.V, M.L.L, R.V, S.D, S.D’M.P, C.T, M. M, P. P, N.M. S.M.A, S.N, M.R.S, R.V, T. S, R.A.B, O.S, N.H, T.Z.T, J.P.T, L.A.A, O.P, M.A.L, J.B.L. Writing, review, and/or revision of the manuscript: A.S.T.E, K.W-L, M.L.L, J.P.T, O.P, R.V, M.A.L, J.B.L. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): R.V, S.N, L.H, G.G, M.R.S, T.S, R.A.B, O.S, N.H, L.A.A, O.P, M.A.L, J.B.L. Study supervision: M.A.L, J.B.L.
Declaration of Interests
J.B.L. is founder and shareholder of BerGenBio ASA. K.W.L., G.G. and J.B.L. are former or current employees of BerGenBio ASA. J.P.T. is scientific founder and CSO of Biocheetah Pte Ltd Singapore and consultant/shareholder Biosyngen Pte Lte Limited and ACTgenomics Taipei Taiwan. S.C. and R.A.B. signed Sponsored Research Agreements with BerGenBio ASA related to a separate research project. The remaining authors declare no conflict of interest.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101649.
Contributor Information
Mark A. LaBarge, Email: mlabarge@coh.org.
James B. Lorens, Email: jim.lorens@uib.no.
Supplemental Information
References
- Amir El A.D., Davis K.L., Tadmor M.D., Simonds E.F., Levine J.H., Bendall S.C., Shenfeld D.K., Krishnaswamy S., Nolan G.P., Pe'er D. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony J., Tan T.Z., Kelly Z., Low J., Choolani M., Recchi C., Gabra H., Thiery J.P., Huang R.Y. The GAS6-AXL signaling network is a mesenchymal (Mes) molecular subtype-specific therapeutic target for ovarian cancer. Sci. Signal. 2016;9:ra97. doi: 10.1126/scisignal.aaf8175. [DOI] [PubMed] [Google Scholar]
- Bachelard-Cascales E., Chapellier M., Delay E., Pochon G., Voeltzel T., Puisieux A., Caron De Fromentel C., Maguer-Satta V. The CD10 enzyme is a key player to identify and regulate human mammary stem cells. Stem Cells. 2010;28:1081–1088. doi: 10.1002/stem.435. [DOI] [PubMed] [Google Scholar]
- Billaud M., Santoro M. Is co-option a prevailing mechanism during cancer progression? Cancer Res. 2011;71:6572–6575. doi: 10.1158/0008-5472.CAN-11-2158. [DOI] [PubMed] [Google Scholar]
- Blanpain C., Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blick T., Hugo H., Widodo E., Waltham M., Pinto C., Mani S.A., Weinberg R.A., Neve R.M., Lenburg M.E., Thompson E.W. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J. Mammary Gland Biol. Neoplasia. 2010;15:235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- Breitling R., Armengaud P., AmtmannHerzyk A.P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Chen L., Jenjaroenpun P., Pillai A.M., Ivshina A.V., Ow G.S., Efthimios M., Zhiqun T., Tan T.Z., Lee S.C., Rogers K. Transposon insertional mutagenesis in mice identifies human breast cancer susceptibility genes and signatures for stratification. Proc. Natl. Acad. Sci. U S A. 2017;114:E2215–E2224. doi: 10.1073/pnas.1701512114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C., Shah S.P., Chin S.F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsen K.T., Haaland G.S., Lie M.K., Lorens J.B., Engelsen A.S.T. The role of Axl receptor tyrosine kinase in tumor cell plasticity and therapy resistance. In: Akslen L.A., Watnick R.S., editors. Biomarkers of the Tumor Microenvironment: Basic Studies and Practical Applications. Switzwerland. Springer Press; 2017. pp. 351–376. [Google Scholar]
- Ding K., Banerjee A., Tan S., Zhao J., Zhuang Q., Li R., Qian P., Liu S., Wu Z.S., Lobie P.E., Zhu T. Artemin, a member of the glial cell line-derived neurotrophic factor family of ligands, is HER2-regulated and mediates acquired trastuzumab resistance by promoting cancer stem cell-like behavior in mammary carcinoma cells. J. Biol. Chem. 2014;289:16057–16071. doi: 10.1074/jbc.M113.529552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C., Spike B.T., Harrell J.C., Johns C., Trejo C.L., Southard-Smith E.M., Perou C.M., Wahl G.M. Sox10 regulates stem/progenitor and mesenchymal cell states in mammary epithelial cells. Cell Rep. 2015;12:2035–2048. doi: 10.1016/j.celrep.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirew P., Stingl J., Raouf A., Turashvili G., Aparicio S., Emerman J.T., Eaves C.J. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat. Med. 2008;14:1384–1389. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- Fridriksdottir A.J., Villadsen R., Morsing M., Klitgaard M.C., Kim J., Petersen O.W., Ronnov-Jessen L. Proof of region-specific multipotent progenitors in human breast epithelia. Proc. Natl. Acad. Sci. U S A. 2017;114:E10102–E10111. doi: 10.1073/pnas.1714063114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe J.C., Pepin F., Pelissier F.A., Sputova K., Fridriksdottir A.J., Guo D.E., Villadsen R., Park M., Petersen O.W., Borowsky A.D. Accumulation of multipotent progenitors with a basal differentiation bias during aging of human mammary epithelia. Cancer Res. 2012;72:3687–3701. doi: 10.1158/0008-5472.CAN-12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraddi R.R., Chung C.Y., Heinz R.E., Balcioglu O., Novotny M., Trejo C.L., Dravis C., Hagos B.M., Mehrabad E.M., Rodewald L.W. single-cell transcriptomes distinguish stem cell state changes and lineage specification programs in early mammary gland development. Cell Rep. 2018;24:1653–1666 e7. doi: 10.1016/j.celrep.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerdrum C., Tiron C., Hoiby T., Stefansson I., Haugen H., Sandal T., Collett K., Li S., Mccormack E., Gjertsen B.T. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. U S A. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette M.A., Duhamel S., Aubert L., Pelletier A., Savage P., Thibault M.P., Johnson R.M., Carmeliet P., Basik M., Gaboury L. The receptor tyrosine kinase AXL is required at multiple steps of the metastatic cascade during HER2-positive breast cancer progression. Cell Rep. 2018;23:1476–1490. doi: 10.1016/j.celrep.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Guo W., Keckesova Z., Donaher J.L., Shibue T., Tischler V., Reinhardt F., Itzkovitz S., Noske A., Zurrer-Hardi U., Bell G. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland G.S., Falk R.S., Straume O., Lorens J.B. Association of warfarin use with lower overall cancer incidence among patients older than 50 years. JAMA Intern. Med. 2017;177:1774–1780. doi: 10.1001/jamainternmed.2017.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.J., Pan A., Franci C., Hu Y., Chang B., Li W., Duan M., Torneros A., Yu J., Heckrodt T.J. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- Hu Y., Smyth G.K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Hugo W., Zaretsky J.M., Sun L., Song C., Moreno B.H., Hu-Lieskovan S., Berent-Maoz B., Pang J., Chmielowski B., Cherry G. Genomic and transcriptomic features of response to anti-pd-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y., Macara I.G. The Par3-like polarity protein Par3L is essential for mammary stem cell maintenance. Nat. Cell Biol. 2014;16:529–537. doi: 10.1038/ncb2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela T.A., Engelsen A.S.T., Rybicka A., Pelissier Vatter F.A., Garbe J.C., Miyano M., Tiron C., Ferariu D., Akslen L.A., Stampfer M.R. Microenvironment-induced non-sporadic expression of the AXL and cKIT receptors are related to epithelial plasticity and drug resistance. Front. Cell Dev. Biol. 2018;6:41. doi: 10.3389/fcell.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P.A., Di Grappa M.A., Khokha R. Active allies: hormones, stem cells and the niche in adult mammopoiesis. Trends Endocrinol. Metab. 2012;23:299–309. doi: 10.1016/j.tem.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Kang J., Perry J.K., Pandey V., Fielder G.C., Mei B., Qian P.X., Wu Z.S., Zhu T., Liu D.X., Lobie P.E. Artemin is oncogenic for human mammary carcinoma cells. Oncogene. 2009;28:2034–2045. doi: 10.1038/onc.2009.66. [DOI] [PubMed] [Google Scholar]
- Kirane A., Ludwig K.F., Sorrelle N., Haaland G., Sandal T., Ranaweera R., Toombs J.E., Wang M., Dineen S.P., Micklem D. Warfarin blocks Gas6-mediated Axl activation required for pancreatic cancer epithelial plasticity and metastasis. Cancer Res. 2015;75:3699–3705. doi: 10.1158/0008-5472.CAN-14-2887-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D.A., Bhakta N.R., Kessenbrock K., Prummel K.D., Yu Y., Takai K., Zhou A., Eyob H., Balakrishnan S., Wang C.Y. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.H., Simonds E.F., Bendall S.C., Davis K.L., Amir E.D., Tadmor M.D., Litvin O., Fienberg H.G., Jager A., Zunder E.R. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew E.D., Oh J., Burrola P.G., Lax I., Zagorska A., Traves P.G., Schlessinger J., Lemke G. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife. 2014;3:e03385. doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja A.M., Rodilla V., Huyghe M., Hannezo E., Landragin C., Renaud O., Leroy O., Rulands S., Simons B.D., Fre S. Clonal analysis of Notch1-expressing cells reveals the existence of unipotent stem cells that retain long-term plasticity in the embryonic mammary gland. Nat. Cell Biol. 2018;20:677–687. doi: 10.1038/s41556-018-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E., Vaillant F., Wu D., Forrest N.C., Pal B., Hart A.H., Asselin-Labat M.L., Gyorki D.E., Ward T., Partanen A. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Lotsberg M.L., Wnuk-Lipinska K., Terry S., Tan T.Z., Lu N., Trachsel-Moncho L., Rosland G.V., Siraji M.I., Hellesoy M., Rayford A. AXL targeting abrogates autophagic flux and induces immunogenic cell death in drug-resistant cancer cells. J. Thorac. Oncol. 2020;15:973–999. doi: 10.1016/j.jtho.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- Ludwig K.F., Du W., Sorrelle N.B., Wnuk-Lipinska K., Topalovski M., Toombs J.E., Cruz V.H., Yabuuchi S., Rajeshkumar N.V., Maitra A. Small-molecule inhibition of Axl targets tumor immune suppression and enhances chemotherapy in pancreatic cancer. Cancer Res. 2018;78:246–255. doi: 10.1158/0008-5472.CAN-17-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell A.J., Stanger B.Z. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 2016;17:413–425. doi: 10.1038/nrm.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A.S., Miller M.A., Gertler F.B., Lauffenburger D.A. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci. Signal. 2013;6:ra66. doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Gomes A.M., Standlee C.R., Rojo M.D., Carmeliet P., Lin Z., Machado H.L. Gas6 is dispensable for pubertal mammary gland development. PLoS One. 2018;13:e0208550. doi: 10.1371/journal.pone.0208550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Pal B., Bouras T., Shi W., Vaillant F., Sheridan J.M., Fu N., Breslin K., Jiang K., Ritchie M.E., Young M. Global changes in the mammary epigenome are induced by hormonal cues and coordinated by Ezh2. Cell Rep. 2013;3:411–426. doi: 10.1016/j.celrep.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Pelissier F., Claudet I., Pelissier-Alicot A.L., Franchitto N. Parental cannabis abuse and accidental intoxications in children: prevention by detecting neglectful situations and at-risk families. Pediatr. Emerg. Care. 2014;30:862–866. doi: 10.1097/PEC.0000000000000288. [DOI] [PubMed] [Google Scholar]
- Pelissier Vatter F.A., Schapiro D., Chang H., Borowsky A.D., Lee J.K., Parvin B., Stampfer M.R., Labarge M.A., Bodenmiller B., Lorens J.B. High-dimensional phenotyping identifies age-emergent cells in human mammary epithelia. Cell Rep. 2018;23:1205–1219. doi: 10.1016/j.celrep.2018.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O.W., Polyak K. Stem cells in the human breast. Cold Spring Harb. Perspect. Biol. 2010;2:a003160. doi: 10.1101/cshperspect.a003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S., Prat A., Sedic M., Proia T., Wronski A., Mazumdar S., Skibinski A., Shirley S.H., Perou C.M., Gill G. Cell-state transitions regulated by SLUG are critical for tissue regeneration and tumor initiation. Stem Cell Rep. 2014;2:633–647. doi: 10.1016/j.stemcr.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Simonds E.F., Bendall S.C., Gibbs K.D., Jr., Bruggner R.V., Linderman M.D., Sachs K., Nolan G.P., Plevritis S.K. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol. 2011;29:886–891. doi: 10.1038/nbt.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios A.C., Fu N.Y., Lindeman G.J., Visvader J.E. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014;506:322–327. doi: 10.1038/nature12948. [DOI] [PubMed] [Google Scholar]
- Sandahl M., Hunter D.M., Strunk K.E., Earp H.S., Cook R.S. Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC Dev. Biol. 2010;10:122. doi: 10.1186/1471-213X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M., Vaillant F., Simpson K.J., Stingl J., Smyth G.K., Asselin-Labat M.L., Wu L., Lindeman G.J., Visvader J.E. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike B.T., Engle D.D., Lin J.C., Cheung S.K., La J., Wahl G.M. A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell Stem Cell. 2012;10:183–197. doi: 10.1016/j.stem.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H.I., Eaves C.J. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Tan T.Z., Miow Q.H., Miki Y., Noda T., Mori S., Huang R.Y., Thiery J.P. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med. 2014;6:1279–1293. doi: 10.15252/emmm.201404208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry S., Abdou A., Engelsen A.S.T., Buart S., Dessen P., Corgnac S., Collares D., Meurice G., Gausdal G., Baud V. AXL targeting overcomes human lung cancer cell resistance to NK- and CTL-mediated cytotoxicity. Cancer Immunol. Res. 2019;7:1789–1802. doi: 10.1158/2326-6066.CIR-18-0903. [DOI] [PubMed] [Google Scholar]
- Vaillant F., Asselin-Labat M.L., Shackleton M., Forrest N.C., Lindeman G.J., Visvader J.E. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- van Keymeulen A., Rocha A.S., Ousset M., Beck B., Bouvencourt G., Rock J., Sharma N., Dekoninck S., Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Villadsen R., Fridriksdottir A.J., Ronnov-Jessen L., Gudjonsson T., Rank F., Labarge M.A., Bissell M.J., Petersen O.W. Evidence for a stem cell hierarchy in the adult human breast. J. Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J.E., Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28:1143–1158. doi: 10.1101/gad.242511.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoriluoto K., Haugen H., Kiviluoto S., Mpindi J.P., Nevo J., Gjerdrum C., Tiron C., Lorens J.B., Ivaska J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- Wuidart A., Sifrim A., Fioramonti M., Matsumura S., Brisebarre A., Brown D., Centonze A., Dannau A., Dubois C., Van Keymeulen A. Early lineage segregation of multipotent embryonic mammary gland progenitors. Nat. Cell Biol. 2018;20:666–676. doi: 10.1038/s41556-018-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Tam W.L., Shibue T., Kaygusuz Y., Reinhardt F., Ng Eaton E., Weinberg R.A. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lee J.C., Lin L., Olivas V., Au V., Laframboise T., Abdel-Rahman M., Wang X., Levine A.D., Rho J.K. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CyTOF data reported in this paper is accessible through Mendeley: https://doi.org/10.17632/j7mrbgt3hh.1. The BeadChip array data of sorted cells from B6.129P2-Axltm1Dgen/J mice are accessible through GEO database, accession number: GSE156662: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE156662.