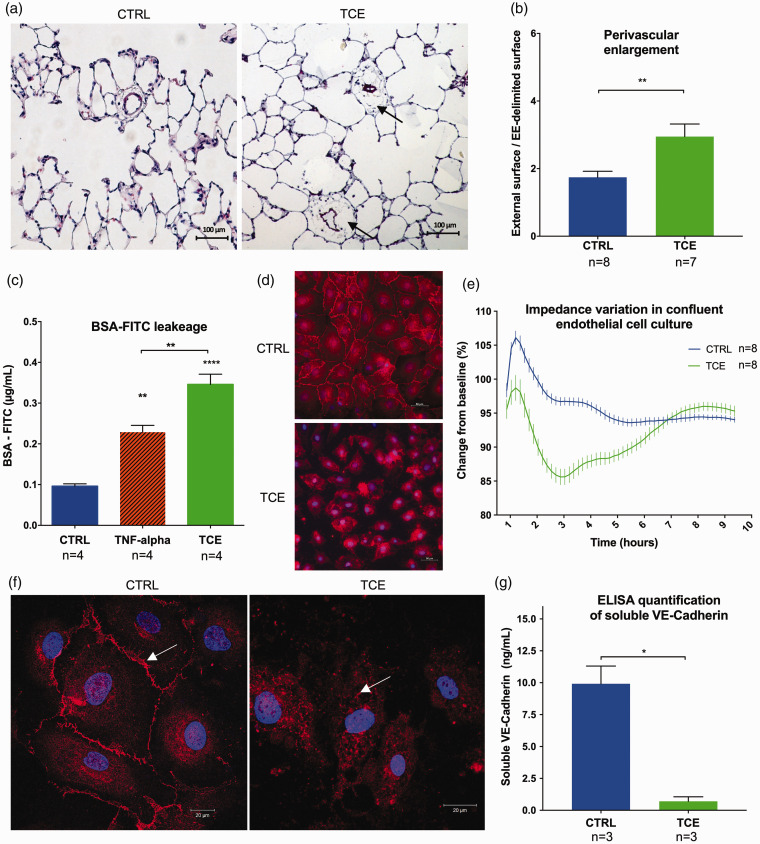
Fig. 1.
Trichloroethylene (TCE)-induced endothelial dysfunction with an increase of vascular permeability. (a) Lung histology 35 days after TCE exposure in rats shows perivascular enlargement (arrows) of pulmonary small distal vessels (diameter ≤50 µm) (Hematoxylin and Eosin stains). (b) Quantification of the perivascular enlargement in control and TCE-exposed lungs. Twenty consecutive fields at 20× magnification were analysed per rat in a blinded fashion. We measured the external surface delimited by the outer edge of the adventitia, and the vascular surface delimited by the external elastica (EE-delimited surface) and assessed the perivascular enlargement through the ratio external surface/EE-delimited surface. (c) BSA-FITC leakage through confluent monolayers of pulmonary endothelial cells is increased after TCE exposure. (d) Immunofluorescence analysis against VE-Cadherin (red) and nuclei (blue): cells exposed to TCE lose adherent junction of VE-Cadherin. (e) Curve of electric impedance (iCELLigence) of confluent monolayers of pulmonary endothelial cells after complete medium change (T0) with TCE (green) or without (blue): TCE induces a reversible decline of electric impedance. (f) Confocal microscopy analysis shows that endothelial cells exposed to TCE lose membrane localisation of VE-Cadherin which is internalised into the cytoplasm. (g) ELISA quantification of soluble VE-Cadherin (reflecting cell membrane expression) in cell culture supernatants of pulmonary endothelial cells with or without 2 h of TCE exposure. A decrease of soluble VE-Cadherin is observed after TCE exposure. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ****p < 0.0001.