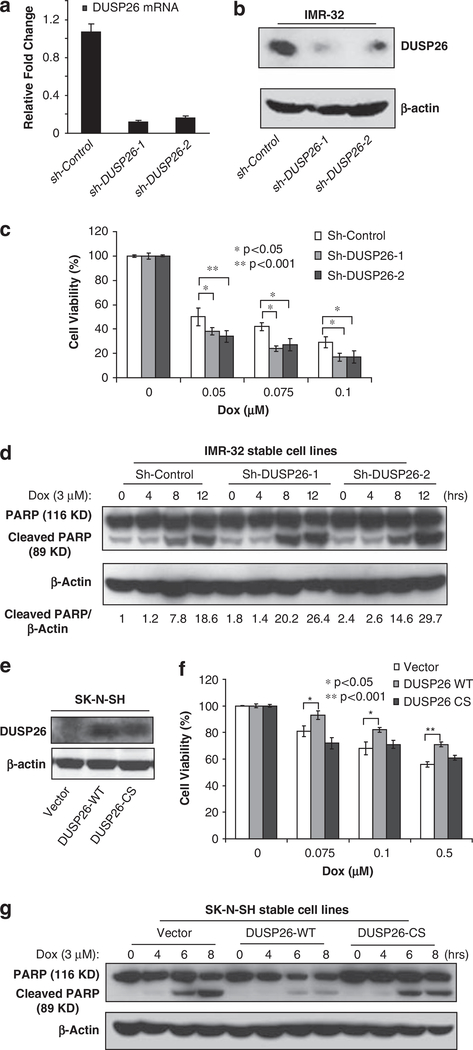
Figure 2.
DUSP26 promotes the resistance of neuroblastoma cells to doxorubincin-induced cytotoxicicity. Knockdown of DUSP26 expression in IMR-32 cells stably transduced with sh-Control and two sh-DUSP26 constructs detected by quantitative reverse transcriptase (RT)-PCR (a) and immunoblotting analysis (b) after 10 days of puromycin (2 μg/ml) selection. (c) The IMR-32 sh-Control, sh-DUSP26–1 and sh-DUSP26–2 cell lines were plated in 96-well plates at 1 × 104 cells per well. After 24 h of growth, all of the cell lines were treated with the indicated μM concentration of doxorubicin for 24 h. Cell viability was determined with the CCK-8 cell viability assay relative to the 0 μM group. All experiments were performed in triplicate and statistical significance was determined by Student’s t-test comparing each sh-DUSP26 with sh-Control group where *P<0.05 and **P<0.001. (d) Knockdown of DUSP26 expression enhances doxorubicin-induced PARP cleavage in neuroblastoma cells. Cells were treated with or without doxorubicin (3 μM) for indicated time points, harvested and subjected to immunoblotting analysis with anti-PARP antibodies to detect the presence of full-length (116 kDa) and cleaved (89 kDa) PARP proteins. The relative level of the cleaved PARP was indicated. (e) DUSP26 expression in SK-N-SH cells stably transduced with pBabe-vector control, DUSP26-WT and DUSP26-CS were analyzed by immunoblotting analysis with anti-DUSP26 antibodies after 10 days of puromycin (2 μg/ml) selection. (f) The SK-N-SH pBabe-vector control, DUSP26-WT and DUSP26-CS cell lines were plated in 96-well plates at 1 × 104 cells per well. After 24 h of growth, all of the cell lines were treated with the indicated μm concentration of doxorubicin for 24 h. Cell viability was determined with the CCK-8 cell viability assay relative to the 0 μm group. All experiments were performed in triplicate and statistical significance was determined by Student’s t-test comparing DUSP26-WT with the vector control group where *P<0.05 and **P<0.001. (g) The SK-N-SH pBabe-vector control, DUSP26-WT and DUSP26-CS cells were seeded into 6-cm dishes for two days. The cells were either left untreated or stimulated with 3 μM doxorubicin for 4, 6 or 8 h. After that, cells were harvested and subjected to immunoblotting analysis with anti-PARP antibodies.