Abstract
Routine postoperative surveillance is recommended for the patients with colorectal cancer (CRC). This study aimed to clarify the conditions indicate initial suspicion of CRC recurrence in different preoperative serum carcinoembryonic antigen (CEA) level groups, including positive physical signs/symptoms, elevated CEA level, positive radiologic studies results, and other elevated tumor markers.
A total of 2268 patients with recurrence after curative surgery for CRC were enrolled in this study. The patients were classified into 3 groups according to preoperative serum CEA level (low, <2 ng/mL; intermediate, ≥2 and <5 ng/mL; and high, ≥5 ng/mL).
Up to 63.6% of recurrence was suspected based on elevated CEA level in the high preoperative serum CEA level group. Patients in the low preoperative serum CEA level group had a higher rate of initial suspicion of recurrence based on positive physical signs or symptoms (36.7% vs 26.9% vs 20.4%, P < .001) and positive radiologic findings (51.4% vs 40.7% vs 29.5%, P < .001) than those in the intermediate and high preoperative serum CEA groups.
Conditions indicate initial suspicion of recurrence varied in the different preoperative serum CEA level groups. In patients with low preoperative serum CEA level, the detection of recurrence depend on abnormal CEA level is less sensitive than intermediate and high preoperative serum CEA groups. We suggest that the strategy for CRC surveillance should not depend on serum CEA level alone. The signs or symptoms of patients, changes in postoperative serial CEA level, and ongoing radiologic or imaging findings must be cautiously monitored.
Keywords: carcinoembryonic antigen, colorectal cancer, recurrence, surveillance
1. Introduction
The use of carcinoembryonic antigen (CEA) level as a tumor marker of colorectal cancer (CRC) was first introduced by Gold et al[1] in 1965. The importance of CEA level follow-up is proven in literature reviews. Routine measurement of preoperative CEA levels and continuous postoperative CEA level surveillance were recommended by both the National Comprehensive Cancer Network and European Society of Medical Oncology guidelines.[2,3] The combination of CEA and other radiologic examinations or imaging studies was widely used in detecting tumor recurrence during postoperative surveillance of CRC. The Follow-up After Colorectal Surgery trial, which was published in 2014, has reported that regular blood CEA level surveillance every 3 to 6 months for 5 years and computer tomography (CT) scan every 12 to 18 months can lead to the earlier detection of CRC recurrence.[4]
In general, for patients with CRC who presented with elevated preoperative CEA level after curative resection, their CEA level usually returned to normal range. When CEA level is elevated again during surveillance, recurrent CRC could be suspected, and radiologic examination or imaging studies, such as CT or positron emission tomography (PET), are conducted for a definite diagnosis of recurrence.
Change in postoperative CEA level is not the only reason for the suspicion of recurrence. Other reasons, including positive physical signs and symptoms, elevated level of other tumor markers, and positive radiologic examination or imaging studies, provide clues for physicians in detecting recurrence during regular follow-up. For the postoperative surveillance of CRC, no previous study had discussed about the reasons for the suspicion of recurrence. Thus, this study aimed to assess the conditions indicate initial suspicion of CRC recurrence, ratio of abnormal serum CEA level at recurrence, definitive criterion used to confirm recurrence in different preoperative serum CEA level groups, and prognostic factors for post recurrence survival.
2. Materials and methods
2.1. Patients
This study was approved by the institutional review board. Data about clinical variables and follow-up status were retrieved from a CRC registry of a single medical center between January 2000 and December 2013. Each patient underwent standard oncological resection of colorectal tumors with open or laparoscopic methods. Demographic, surgical, pathologic, operative, and follow-up data were also collected. A total of 11,709 consecutive patients with stage I–III CRC underwent curative surgery. However, 109 patients with surgical mortality, 280 patients with recurrence within 6 months post curative survey, and 171 patients without data on preoperative or postoperative serum CEA level were excluded. Patients were classified into 3 different groups according to preoperative serum CEA level (low, CEA level <2 ng/mL; intermediate, CEA level ≥2 and <5 ng/mL; and high, CEA level ≥5 ng/mL). Finally, 2268 patients with tumor recurrence, including local recurrence and systemic recurrence, after curative surgery for CRC were enrolled in this study. A flow chart of patients enrolled in this study was presented in Figure 1.
Figure 1.
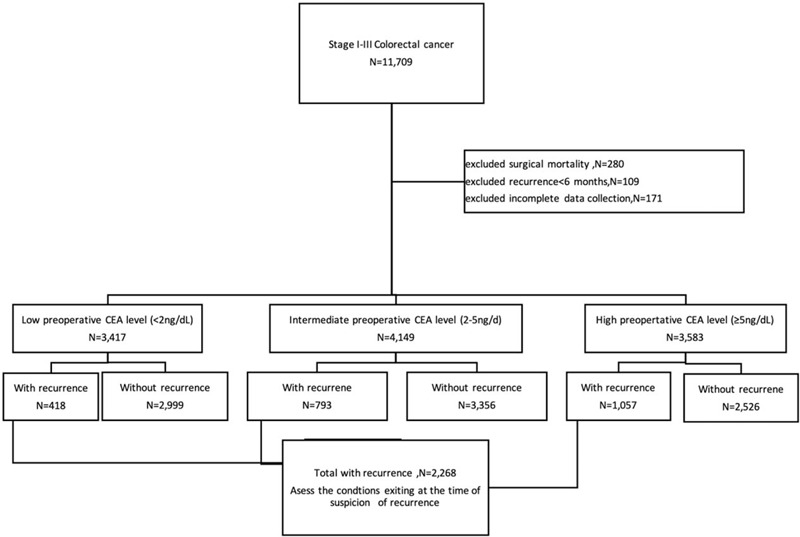
Flow chart of 2268 colorectal cancer patients with recurrence during postoperative surveillance. CEA = carcinoembryonic antigen, N = number.
Patient-related variables consisted of age and gender. The patients were divided into 2 groups according to age: <65 years (young) and ≥65 years (old). Tumor-related factors consisted of location, size, morphology, histology, degree of differentiation, and stage. Tumor stages were determined according to the 7th edition of the Union for International Cancer Control-American Joint Committee on Cancer tumor, node, metastasis staging system.[5] Tumor location was categorized as colon and rectum. Tumor morphology was divided into polypoid (flat and polypoid tumors), ulcerative, and infiltrative tumors. Recurrence detected within 3 years after curative surgery was defined as early recurrence, whereas recurrence identified more than 3 years after curative surgery was considered as late recurrence.
2.2. Follow-up
Regular surveillance program was performed for all patients. Patients were followed-up routinely at 3-month intervals for the first 2 years and at 6-month intervals thereafter and every 12 months after 5 years. The follow-up programs included physician inquiry, psychical examinations, and assessment of CEA level at every visit. Liver echo plus chest radiography and colonoscopy were performed annually for at least 5 years. CT scan from chest to pelvis was carried out for at least 3 years in high-risk patients or when a patient presented with abnormal imaging findings or signs and symptoms. If an elevated postoperative CEA level was found, would recheck the CEA level within 1 to 2 months. All patients with CEA elevation were followed carefully to make sure no evidence of recurrence.
The conditions at initial suspicion of recurrence were recorded, which included elevated CEA level, physical signs or symptoms, positive imaging findings, and elevated levels of other tumor markers, such as carbohydrate antigen 19–9 or cancer antigen 125. Recurrent CEA (rCEA) was defined as an CEA level at the initial suspicion of recurrence.
Definite recurrence was defined based on the following criteria:
-
(1)
locoregional recurrence on histological examination,
-
(2)
liver or lung metastases on imaging studies,
-
(3)
bone metastases based on presenting symptoms plus imaging or bone scan results,
-
(4)
systemic lymph node metastases on histologic examination or imaging studies,
-
(5)
intraabdominal or peritoneal recurrence based on positive cytology or histology results, and
-
(6)
brain metastases on histology examination or presenting symptoms plus positive imaging findings.
2.3. Statistical analysis
Post recurrence survival was defined as the duration between the date of confirmed recurrence and the date of last follow-up. The last follow-up date was on December 2016. The median follow-up time was 66.5 months. All analyses were performed using the Statistical Package for the Social Sciences software version 11.0 (SPSS Inc. Chicago, IL). Analysis of variance test and χ2 test were utilized for continuous variables and categorical variables. A multivariate binary logistic regression model was also used to determine the independent associations between the characteristics of patients. The post-recurrence overall survival (OS) were analyzed using the Kaplan–Meier method. The significance of the prognostic variables was analyzed with Cox multivariate proportional hazards model. Statistical significance was set at P < .05 (OS: the proportion of patients with cancer who survived a specified time interval after surgery).
3. Results
3.1. Patient characteristics
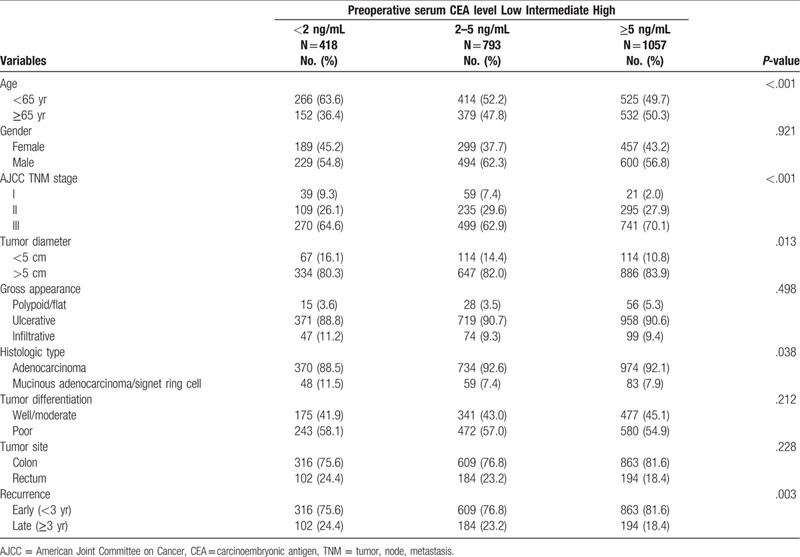
The clinicopathological characteristics of the 2268 patients categorized based on preoperative serum CEA level are presented in Table 1. The proportions of patients aged >65 years (50.3% vs 47.8% vs 36.4%, P < .001), those with stage III cancer (70.1% vs 62.9% vs 54.8%, P < .001), and those with larger tumor size >5 cm (83.9% vs 82.0% vs 80.3%, P = .013) were significant higher in the high preoperative CEA level group than in the intermediate and low CEA level groups. The proportions of mucinous/signet ring cell content (11.5% vs 7.4% vs 7.9%, P = .038) and late recurrence (24.4% vs 23.2% vs 18.4%, P = .003) was significantly higher in the low preoperative CEA level group than in the intermediate and high CEA level groups. The proportions of sex, tumor gross appearance, tumor histologic grading, and tumor site were similar in the 3 groups.
Table 1.
Clinicopathological characteristics of the 2268 patients with colorectal cancer who had recurrence after curative surgery.
3.2. CEA level at recurrence
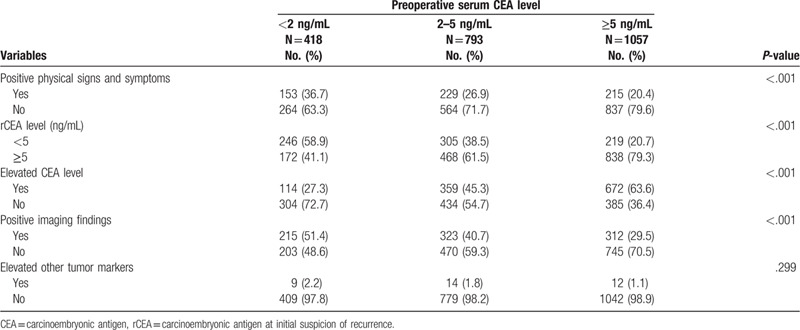
Among the 418 patients with recurrence who had low preoperative serum CEA level, the rCEA level was ≥5 ng/mL in 264 (63.1%) patients. Among the 793 patients with recurrence who had intermediate preoperative serum CEA level, the rCEA level was ≥5 ng/mL in 564 (71.1%) patients. Among the 1057 patients with recurrence who had high preoperative serum CEA level, the rCEA level was ≥5 ng/mL in 837 (79.2%) patients. The proportion of rCEA level ≥5 ng/mL in patients with low preoperative serum CEA level was significantly lower (P < .001).
3.3. Conditions at initial suspicion of recurrence
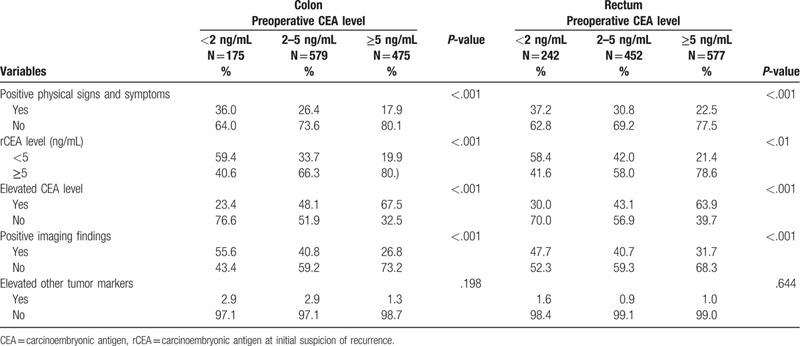
Suspicion of recurrence based on elevated CEA level during follow-up was significantly different (P < .001) in the 3 preoperative serum CEA level groups. In the low preoperative serum CEA level group, only 27.3% of recurrence was suspected based on changes in CEA level during follow-up. However, up to 45.3% to 63.6% of recurrence was suspected according to changes in CEA level in the intermediate and high preoperative serum CEA level groups. The low preoperative serum CEA level group had higher rate of recurrence suspicion according to positive physical signs or symptoms (36.7% vs 26.9% vs 20.4%, P < .001) and positive imaging findings (51.4% vs 40.7% vs 29.5%, P < .001) than the intermediate and high preoperative serum CEA level groups (Table 2).
Table 2.
Conditions indicate initial suspicion of recurrence of the 2268 patients with colorectal cancer who had recurrence after curative surgery.
We further classified the subgroup into the early recurrence group (recurrence within 3 years) and late recurrence group (recurrence after 3 years), as shown in Table 3. For patients with high preoperative serum CEA level, suspicion of recurrence based on elevated CEA level was significantly higher in both the early and late recurrence groups. For patients with low preoperative serum CEA level, suspicion of recurrence based on positive symptoms and radiologic findings was significantly higher in both the early and late recurrence groups. The results were similar in the subgroups with colon and rectal cancer (Table 4).
Table 3.
Conditions indicate initial suspicion of recurrence: early recurrence vs late recurrence.
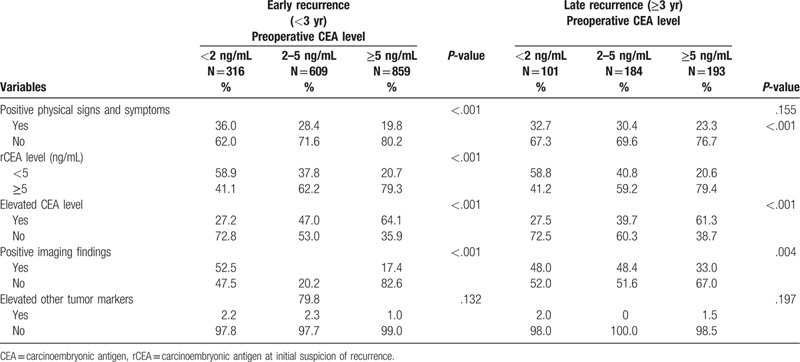
Table 4.
Conditions indicate initial suspicion of recurrence: colon versus rectum.
3.4. Criterion in confirming recurrence
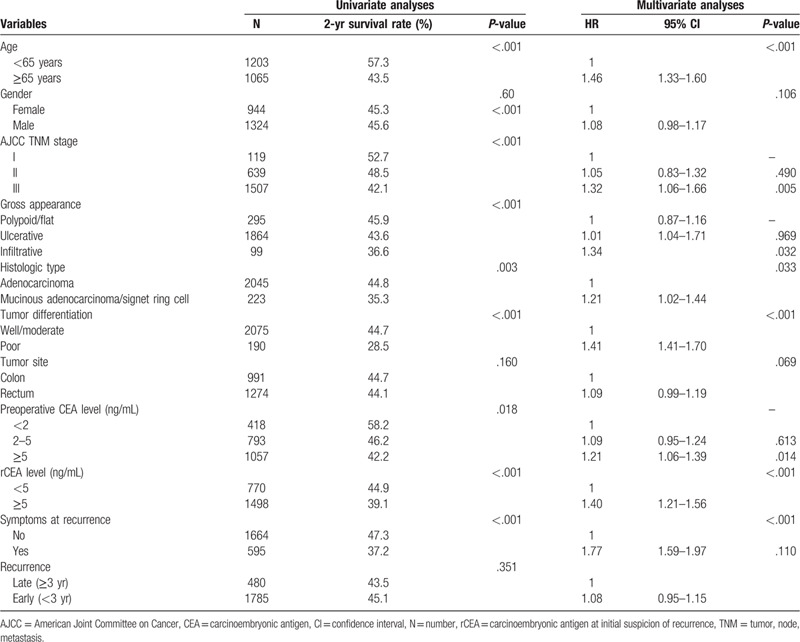
The most definitive criterion in confirming recurrence is presented in Table 5. Most recurrences were confirmed according to positive abdominal or pulmonary CT findings (63.8%), and no significant difference was observed among the 3 groups. The different variables associated with post-recurrence are listed in Table 6, and old age (>65 years), American Joint Committee on Cancer staging (stage III), gross infiltrative appearance, tumor with poor differentiation, preoperative CEA ≥5 ng/mL, rCEA level ≥5 ng/mL, and positive symptoms at recurrence were associated with shorter post recurrence OS in multivariate analysis.
Table 5.
Most definitive criterion used to confirm progression of the 2268 patients.
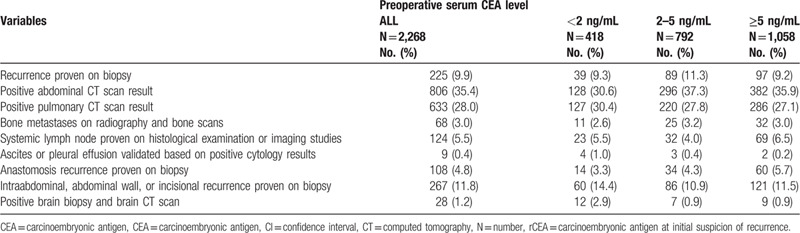
Table 6.
Cox proportion hazard models on post recurrence overall survival of the 2268 patients.
4. Discussion
Despite the much progress of surgical methods, team care, adjuvant chemotherapy, and target therapy in recent years, the prognosis of cancer recurrence still highly related with timing of recurrence detection. Regular follow-up after surgery due to cancer is widely accepted. The purpose of regular follow-up is for the early detection of recurrence and rapid treatment with surgery or chemotherapy, target therapy, or radiation therapy to prolong survival and improve cancer recurrence-related symptoms. Patients with solitary liver or lung metastasis or resectable intraabdominal recurrence can survive longer after metastasectomy.[6,7] Early intervention of recurrence with systemic chemotherapy or target therapy also improved survival.[8]
Measurement of serum CEA level as a routine surveillance method is relatively convenient and cost-effective for patients with CRC.[9] However, the use of CEA level as the only surveillance tool may highly miss the detection of recurrence. The protocol of CRC surveillance with the combination of CEA level measurement and other imaging studies was widely accepted. However, the image items and time that should be used still remains controversial. Some studies have reported that shorter interval and detailed radiologic items for an intensive follow-up can prolong survival due to the early detection of recurrence and early intervention.[10] In contrast, some studies have reported the lack of survival benefit with intensive interval and use of detailed items after a long-term follow-up. A large-scale randomized study reported by Follow-up After Colorectal Surgery has compared the 3 to 5 years scheduled follow-up of the 3 groups (CEA only, CT only, and CEA + CT) to detect the recurrence of CRC.[4] The result has shown a higher risk of recurrence in the combined CEA + CT group. However, the OS rate was not statistically significant. They have suggested that the use of CEA + CT is not beneficial during late follow-up. In actual clinical settings, the benefit decreased with time due to the incidence of recurrence that reduced annually. Most physicians will prolong the follow-up interval after 5 years. Radiologic examination items were also decreased, and some were not used if the tumor markers were not elevated. We agree to a more intense follow-up program within the first 3 years after surgery. Moreover, we did not believe that surveillance with CT scan or other imaging studies is not necessary after 3 years. In our study, 21.2% of recurrence still occurred after 3 years, and up to 72.5% of recurrence could not be detected with elevated CEA levels during late follow-up in the low preoperative serum CEA level group.
More sensitive imaging, such as PET, may help in the earlier detection of metastasis rather than the measurement of CEA level along with radiologic examination if performed regularly.[11] However, a PET scan is still more expensive than a traditional radiologic exam, which is not covered by most health insurance and has a limited availability. Therefore, it is not usually recommended as the first-line surveillance tool. However, PET or PET-CT should be considered as second-line tool if clinical or laboratory data along with negative or equivocal traditional radiologic findings are suggestive of recurrence.[12]
The high preoperative CEA level group had a higher recurrence rate, and early detection of recurrence is easier in this group owing to the elevated CEA level before abnormal symptoms or radiologic findings can be observed during surveillance. In contrast, patients with low preoperative CEA level have a better prognosis. Frequent radiologic examination is not cost-effective. However, the low sensitivity of CEA level when used in surveillance usually causes delay in the diagnosis of recurrence. In 1 Japanese study that included 106 patients with CRC who presented with recurrence after curative surgery, only 55.9% of patients with normal CEA at initial diagnosis had a serum CEA level ≥5 ng/mL at recurrence.[13] In our study, up to 33.9% of patients with recurrence had normal rCEA level. A cutoff value of 5 ng/mL decreases the sensitivity rate of recurrence detection. In the high preoperative serum CEA level group, 79.3% of patients had abnormal rCEA level. However, in the low preoperative serum CEA level group, only 41.1% had an abnormal rCEA level. Surveillance using CEA level alone is risky as it might miss the detection of both early or late recurrence in this special group of patients. Other tumor markers, such as carbohydrate antigen 19–9 and cancer antigen 125, could also be useful in surveillance, particularly in some patients with CRC who did not have an elevated CEA level before surgery or during follow-up.[14–16] The use of other tumor markers could increase the sensitivity and specificity of the detection of CRC recurrence.
Most studies have suggested that a CEA cutoff value of 5 ng/dL should be used. However, according to our result, in patients with low preoperative CEA level, a low cutoff value and trend in the series of examinations were more important when recurrence was suspected. Previous studies have suggested that the use of serial CEA level measurements rather than 1 CEA level measurement should be further investigated.[17,18] The different cutoff values for CEA were also discussed before. A high cutoff value increases the specificity for the detection of recurrence but decreases sensitivity.[19]
A high rCEA level was associated with shorter post recurrence OS in this study. In relation to this, the possible mechanism is increased CEA secretion from more metastatic tumor loading or more advanced tumor behavior. This finding is consistent with those of previous studies.[20,21] Bhatti has reported a significantly lower portion of mastectomy and significantly poor survival in patients with high CEA level at recurrence.[22]
There is 1 limitation for this study. The study design is based on recurrent cases only, not a cohort-design study. We analyzed the reasons why recurrence was initial suspected only from the definite recurrence patients. For patients without recurrence during surveillance, they could also present with abnormal symptoms or elevated CEA level. The rate is not clear in this study.
5. Conclusion
In the present study, we examined the conditions indicate recurrence during the surveillance of patients with CRC after curative surgery and its association with preoperative serum CEA level. We found that the conditions varied in the different preoperative serum CEA level groups. In patients with low preoperative serum CEA level, the detection of recurrence depend on abnormal CEA level is less sensitive than intermediate and high preoperative serum CEA groups. The cause of suspicion for recurrence based on changes in CEA level is 63.6% in the high preoperative serum CEA level group and only 27.3% in the low preoperative serum CEA level group. Using serum CEA level as only surveillance tool has risk of delayed recurrence detection. We suggest that the strategy for CRC surveillance should not depend on serum CEA level alone. The signs or symptoms of patients, changes in postoperative serial CEA level, and ongoing radiologic or imaging findings must be cautiously monitored.
Author contributions
Conceptualization: Chengchou Lai, Hsin-Yuan Hung, Paoshiu Hsieh, Sumfu Chiang.
Data curation: Chengchou Lai, Hsin-Yuan Hung, Jengfu You, Jyming Chiang, Paoshiu Hsieh, Sumfu Chiang.
Formal analysis: Hsin-Yuan Hung, Jengfu You.
Investigation: Hsin-Yuan Hung, Jyming Chiang, Wensy Tasi.
Methodology: Hsin-Yuan Hung, Jyming Chiang, Wensy Tasi.
Writing – original draft: Hsin-Yuan Hung.
Writing – review & editing: Hsin-Yuan Hung.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CRC = colorectal cancer, CT = Computed tomography, OS = overall survival.
How to cite this article: Hung H, You J, Chiang J, Hsieh P, Chiang S, Lai C, Tasi W, Yeh C. Why recurrence was initially suspected during colorectal cancer postoperative surveillance? A retrospective analysis. Medicine. 2020;99:43(e22803).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med 1965;121:439–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beson AB, 3rd, Venook AP, Bekaii-Saab T, et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw 2014;12:1028–59. [DOI] [PubMed] [Google Scholar]
- [3].Van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer 2014;50:1.e1–34. [DOI] [PubMed] [Google Scholar]
- [4].Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263–70. [DOI] [PubMed] [Google Scholar]
- [5].Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th edNew York: Springer; 2010. [Google Scholar]
- [6].Ambiru S, Miyazaki M, Ito H, et al. Resection of hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer 1998;82:274–8. [DOI] [PubMed] [Google Scholar]
- [7].Shah SA, Haddad R, Al-Sukhni W, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg 2006;202:468–75. [DOI] [PubMed] [Google Scholar]
- [8].Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563–72. [DOI] [PubMed] [Google Scholar]
- [9].Bruinvels DJ, Stiggelbout AM, Kievit J, et al. Follow-up of patients with colorectal cancer: a meta-analysis. Ann Surg 1994;219:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Locker GY, Hamilton S, Harri J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006;24:5313–27. [DOI] [PubMed] [Google Scholar]
- [11].Young PE, Womeldorph CM, Johnson EK, et al. Early detection of colorectal cancer recurrence in patients undergoing surgery with curative intent: current status and challenges. J Cancer 2014;5:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gade M, Kubik M, Fisker RV, et al. Diagnostic value of (18)F-FDG PET/CT as first choice in the detection of recurrent colorectal cancer due to rising CEA. Cancer Imaging 2015;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saito G, Sadahiro S, Kamata H, et al. Monitoring of serum carcinoembryonic antigen levels after curative resection of colon cancer: cutoff values determined according to preoperative levels enhance the diagnostic accuracy for recurrence. Oncology 2017;92:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Filella X, Molina R, Grau JJ, et al. Prognostic value of CA 19.9 levels in colorectal cancer. Ann Surg 1992;216:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Reiter W, Stieber P, Reuter C, et al. Multivariate analysis of the prognostic value of CEA and CA 19-9 serum levels in colorectal cancer. Anticancer Res 2000;20:5195–8. [PubMed] [Google Scholar]
- [16].Quentmeier A, Schlag P, Geisen HP, et al. Evaluation of Ca 12-5 as a tumor marker for gastric and colo-rectal cancer in comparison to CEA and Ca 19-9. Eur J Surg Oncol 1987;13:197–201. [PubMed] [Google Scholar]
- [17].Tan E, Gouvas N, Nicholls RJ, et al. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol 2009;18:15–24. [DOI] [PubMed] [Google Scholar]
- [18].Wichmann MW, Lau-Werner U, Müller C, et al. Carcinoembryonic antigen for the detection of recurrent disease following curative resection of colorectal cancer. Anticancer Res 2000;20:4953–5. [PubMed] [Google Scholar]
- [19].Körner H, Söreide K, Stokkeland PJ, et al. Diagnostic accuracy of serum-carcinoembryonic antigen in recurrent colorectal cancer: a receiver operating characteristic curve analysis. Ann Surg Oncol 2007;14:417–23. [DOI] [PubMed] [Google Scholar]
- [20].Lin JK, Lin CC, Yang SH, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis 2011;26:1135–41. [DOI] [PubMed] [Google Scholar]
- [21].Bakalakos EA, Burak WE, Jr, Young DC, et al. Is carcino-embryonic antigen useful in the follow-up management of patients with colorectal liver metastases? Am J Surg 1999;177:2–6. [DOI] [PubMed] [Google Scholar]
- [22].Bhatti I, Patel M, Dennison AR, et al. Utility of postoperative CEA for surveillance of recurrence after resection of primary colorectal cancer. Int J Surg 2015;16:123–8. [DOI] [PubMed] [Google Scholar]


