Abstract
Rationale:
Although the cancer incidence continues to rise, cancer mortality has declined over the past decade, in large part due to more efficacious chemotherapeutic regimens thus, the ability to use first-line chemotherapeutic agents in the treatment of patients with cancer is crucial. Antineoplastic agents can potentially cause toxic and/or hypersensitivity reactions, that can have serious consequences. Anaphylaxis is a big pitfall in oncological patients; the most important aspect in diagnosing anaphylaxis is to precisely identify the offending agent to prevent future events. Paclitaxel (Taxol) is widely used as antitumor medication in the ovarian, breast, non-small-cell lung, and other cancers. Paclitaxel hypersensitivity reactions are frequently described in the literature, but fatalities are rarely reported. Due to the low solubility of paclitaxel, the compound requires dissolution in Cremophor EL, a derivative of castor oil.
Patient concerns:
A 79-year-old man was affected by high-grade non-papillary urothelial carcinoma and underwent a radical cystectomy and prostatectomy with locoregional lymphadenectomy.
Diagnosis:
Eight months later, relapse was detected, and penis amputation and left nephrostomy were performed. Multiple metastases to lymph nodes were detected.
Interventions:
Palliative chemotherapy was started with Paclitaxel (110 mg) infused at a rate of 50 mL/h. Despite premedication with cetirizine dihydrochloride, dexamethasone, ondansetron, ranitidine, 20 min after Paclitaxel infusion starts, the patient developed general distress, followed by cardiac arrest.
Outcomes:
The mechanism of fatal paclitaxel-associated hypersensitivity reaction is uncertain and its solvent vehicle Cremophor EL may be involved. Several mechanisms have been postulated: an IgE-mediated mast cell degranulation induced by paclitaxel or Cremophor EL, a non-IgE-mediated idiosyncratic mast cell degranulation by paclitaxel or by Cremophor EL, and complement activation. Severe hypersensitivity reactions with fatal outcome are considered rare.
Lessons:
The unpredictability and often dramatic reactions of Taxol cause substantial anxiety for doctors and caretakers. They also represent a significant logistic and financial burden on hospitals. Despite premedication, skin testing, and desensitization protocols administration of taxane-based, chemotherapy cannot be considered safe and severe to fatal hypersensitivity reactions cannot be prevented.
Keywords: case report, cremophor EL, fatal anaphylactic shock, hypersensitivity reaction, mast cells degranulation, paclitaxel, tryptase
1. Introduction
Hypersensitivity reactions (HSR) to paclitaxel were reported soon after the beginning of taxol trials in the early 1980s and kept attracting attention.[1,2] The estimated incidence of HSRs after paclitaxel infusion is <44% for mild HSRs and <10% for severe HSRs while the fatal outcome is rare.[3] Symptoms vary from mild pruritus to severe and life-threatening or fatal anaphylactic reactions. HSRs predominantly occur during the first 10 to 15 min of infusion with 78% within the first 10 min, and in 95% of cases reactions occur during the first or second infusion.[4,5] In a few cases, HSRs occur during subsequent infusions. Paclitaxel HSRs range in severity from urticaria, angioedema, erythematous rashes to systemic anaphylaxis with respiratory arrest, cardiac collapse, and death.[2] Some patients developed skin reactions several days or up to a week after infusion. Between 1997 and 2007 in the United States, Europe, and Japan, 171 cases of anaphylaxis caused by cremophor-containing paclitaxel have been reported.[6] In those reports, 58 (34%) of the patients could not survive the anaphylactic shock. In 22% of fatalities, patients received premedication with corticosteroids before the injection of paclitaxel. Medical Research on Adverse Drug Events and Reports (Med-RADAR) also reviewed 6 cases of cremophor-containing paclitaxel-induced anaphylaxis with two fatal cases.[7] A meta-analysis evaluated the impact of infusion time in cases of anaphylaxis produced by paclitaxel and showed no difference in the risk of developing hypersensitivity when treatment was administered over 3 or 24 h (risk ratio: 1.86; 95% confidence interval: 0.63–5.52).[8] Furthermore, Hainsworth et al noted no difference in activity between 1-day and 3-day paclitaxel schedules in which each dose was administered by1-h infusion.[9] The occurrence of HSRs can be influenced by the administration of an appropriate premedication. Paclitaxel-related immediate HSRs occur in up to 30% of patients, with this percentage decreasing to under 10% with the administration of antihistamine and corticosteroid premedication.[10–13] Kwon et al showed that, compared with a single administration 30 min before treatment, administration of dexamethasone 12 and 6 h before infusion of paclitaxel led to fewer HSRs.[14] Other studies showed no difference.[15] Even though, the use of premedication and/or the slowing of infusion rates are effective but not always successful with a small percentage of patients still developing HSRs despite their intense pre-medication with dexamethasone and antihistamines (diphenhydramine, cimetidine, ranitidine).[16,17] Oral premedication with dexamethasone—a long-acting glucocorticoid with a biologic half-life of ∼48 h and noticeable onset of biologic activity after several hours—at a dose of 20 mg (given orally at 12 and 6 h before infusion of paclitaxel) has been shown to reduce the incidence of paclitaxel-induced HSRs significantly.[18] In fact, dexamethasone strongly inhibits inflammation, especially the cellular-mediated immunity and the production or action of the local mediators of inflammation, such as the prostaglandins and lymphokines. Furthermore, dexamethasone reduces vascular permeability and maintains normal vascular responsiveness to circulating vasoconstrictor factors. However, due to logistical factors, short-course premedication with intravenously administered dexamethasone—given 30 min prior to paclitaxel infusion—has become customary in many centers.[1] In an attempt to reduce steroid-induced side effects, especially for patients receiving weekly paclitaxel, dexamethasone dose reduction has been proposed without an increase of severe HSRs.[19]
Paclitaxel HSRs are frequently described in the literature, but fatalities are rarely reported. We present a fatal paclitaxel-associated anaphylaxis case in which a complete laboratory workup was performed, with a dosage of the sieric tryptase and immunohistochemical positiveness around the mast cells demonstrating evidence of degranulation to prove trigger activation of mast cells. Tryptase is the most abundant secretory granule-derived serine proteinase contained in mast cells and it is considered a reliable marker for mast cell activation.
2. Clinical case
A 79-year-old man was affected by high-grade non-papillary urothelial carcinoma and underwent a radical cystectomy and prostatectomy with locoregional lymphadenectomy. Eight months later, relapse was detected, and penis amputation and left nephrostomy were performed. Multiple metastases to lymph nodes were detected. Palliative chemotherapy was started with Paclitaxel (110 mg) mixed with 500 cm3 physiological solution and infused at a rate of 50 mL/h. Despite premedication with cetirizine dihydrochloride, dexamethasone, ondansetron, ranitidine, 20 min after Paclitaxel infusion starts, the patient presented cough and dyspnea. He rapidly developed general distress, followed by cardiac arrest. Paclitaxel infusion was immediately stopped and intramuscular epinephrine (0.5 mg) injection was performed. Cardiopulmonary resuscitation, which included intubation and respiratory support, was started without delay and remained unsuccessful. A complete post-mortem examination was performed two days after death. Autopsy confirming the presence of right ileostomy, radical cystectomy, and left nephrostomy. Additional findings included pulmonary, pancreatic, and adrenal metastasis. Metastatic lymph nodes at pulmonary hilum were also observed (Fig. 1). The heart was increased in size and volume and coronary arteries examination excluded significant obstruction of the lumen. Pulmonary edema was recorded. Spleen and liver were increased in volume, with regular shape. Histological examination revealed mild cerebral edema and acute pulmonary edema mixed to acute pulmonary emphysema. Myocardial interstitial edema was also detected. An immunohistochemical technique was used to estimate the mast cell population, using the anti-tryptase antibody as a mast cell specific marker. Pulmonary mast cells were identified and a great number of degranulating mast cell with tryptase-positive material outside was observed (Fig. 2). Finally, dosing of the tryptases was performed on a blood sample confirming a concentration of 123.0 μg/L (cut-off value of 45 μg/L for tryptase measured post-mortem has been established for anaphylaxis). This case dealt with a death of judicial interest, for which ethical approval and consent were requested and obtained from the Judicial Authority.
Figure 1.
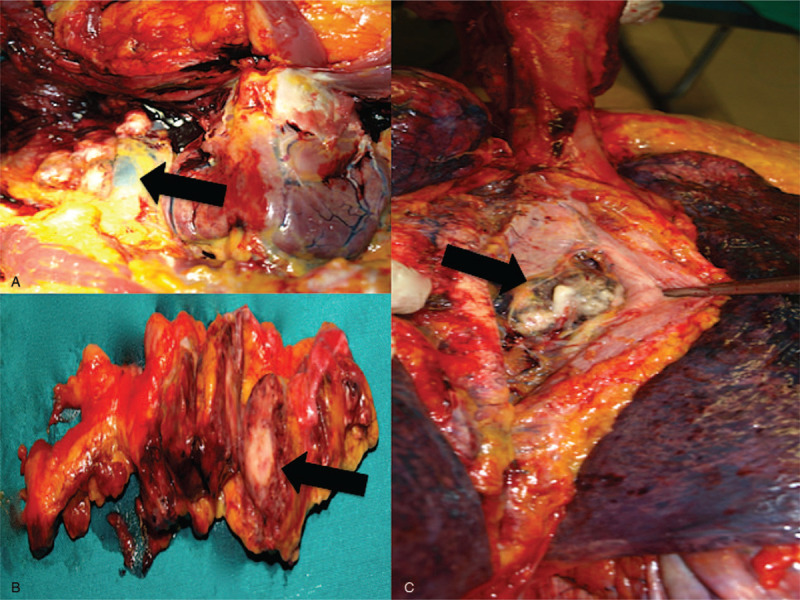
Laterocervical (A), hilar (B), and pancreatic (C) metastases (arrows) of high-grade infiltrating non-papillary urothelial carcinoma treated with radical cystectomy.
Figure 2.
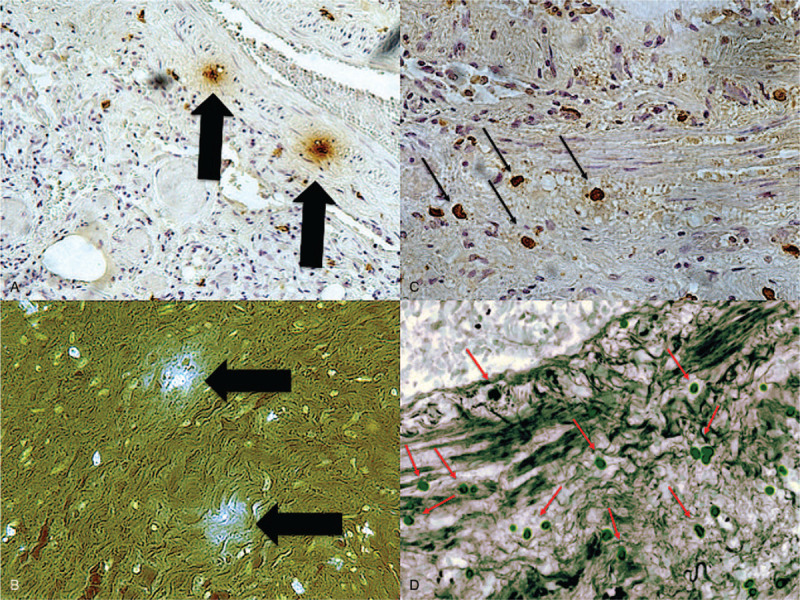
Lung: (A) degranulating mast cells (starry effect in brown, Ab anti-tryptase ×60) with tryptase positive material outside the cells (B) was documented (starry effect in blue, fluorescence ×100). (C) Tryptase positiveness around the mast cells (brown reactions) (Ab anti-tryptase ×40) reveals evidence of mast cells degranulation; an (D) high number of mast cells was documented in the lung (Ab anti-tryptase ×20).
3. Discussion
The mechanism of paclitaxel-associated anaphylaxis is still uncertain. In fact, most HSRs occur within the first minutes of infusion of paclitaxel, usually after the first or second dose, indicating that prior sensitization is not necessary. For this reason, the main role of IgE in the mechanism of anaphylaxis after infusion of paclitaxel was excluded even if recently a case of IgE-mediated reaction to paclitaxel was proved by skin and in vitro testing.[20,21] Macrogolglycerol ricinoleate has also been implicated in anaphylactic reactions on the basis that it can induce complement activation, giving rise to anaphylatoxins that trigger mast cells and basophils for a secretory response.[22,23]
There is substantial evidence suggesting that Cremophor EL (CrEL) may be involved in anaphylaxis induced by paclitaxel.[24,25] Cremophor (CrEL) is a non-ionic emulsifier used to improve the solubility of various poorly water soluble drugs, including paclitaxel consisting of amphiphilic molecules mixture that form micelles in the water acting as a trigger in complement activation.[26] In contrast to earlier reports, CrEL is not an inert vehicle but exerts a range of biological effects, some of which have important clinical implications. The pharmacokinetic behavior of CrEL is dose-independent, although its clearance is highly influenced by the duration of the infusion. This is particularly important since CrEL can affect the disposition of various drugs by changing the unbound drug concentration through micellar encapsulation. Micelles are multimolecular aggregates that usually appear as spherical “core-shell” structures with a dense nucleus surrounded by a less electrodense halo and represent a particulate substance unprotected by surface-bound C regulatory proteins; therefore, satisfying two basic conditions for becoming a C activator. Symptoms induced by cremophor are more likely to be type I HSRs.[27] Activation of complement due to the binding of C3 to their hydrophilic adhesive surface in the absence of immune complex leads to the release of C3a, C5a, and C5b-9, which trigger activation of mast cells, basophils, and other cells via their specific receptors.[28–30] In this case, degranulating mast cells with tryptase positive material outside the cells was documented. Tryptase is the most abundant secretory granule-derived serine proteinase contained in mast cells and it is considered a reliable marker for mast cell activation. Tryptase positiveness around the mast cells reveals evidence of degranulation to demonstrate trigger activation of mast cells.
The concept that C activation by CrEL would underlie HSRs to paclitaxel was based on the demonstration that CrEL fully accounted for C activation by paclitaxel in vitro.[23] Interestingly, there is substantial inter-individual variation, and sensitivity to a specific liposome does not necessarily imply sensitivity to others. HSRs where the allergen can activate C have been tentatively named C activation-related pseudoallergy (CARPA). Symptoms of CARPA are the same as seen in common allergy or classical type I reactions, while others are unique to C activation. Perhaps the most important distinguishing feature of CARPA is the lack of presensitization and reinforcement; the reaction arises at the first exposure to the drug and then it decreases, rather than increases upon repeated exposure. According to Irizarry et al, allergic reactions following the first dose of cremophor-containing drugs can be explained by the wide use of cremophor, which can result in prior sensitization to cremophor.[7] Differently, authors have shown that Cremophor used without paclitaxel and without premedication does not trigger HSRs while paclitaxel alone induced histamine release in 3 healthy controls but cremophor alone did not.[31] It has been observed that similar HSRs have not been reported with a Cremophor-free formulation of paclitaxel that received FDA approval in 2005. Polysorbate 80 was adopted instead of cremophor. Nevertheless, HSRs with polysorbate 80-containing docetaxel have been reported as well.[24,32]
Post-mortem dosing of mast cell tryptase is widely performed in forensic practice for the assessment of cases of suspected anaphylaxis.[33–35] However, despite the extensive use of the method, the interpretation of the results is complex in relation to the presence of confounding factors including the state of conservation of the biological matrix and the increase in the levels of tryptase in other conditions different from anaphylaxis.[36,37]
4. Conclusion
Despite premedication, skin testing, and desensitization protocols administration of taxane-based chemotherapy cannot be considered completely safe and severe to fatal HSRs cannot be prevented. The use of premedication and/or the slowing of infusion rates are effective but not always successful with a small percentage of patients still developing HSRs despite their intense pre-medication with dexamethasone and antihistamines (diphenhydramine, cimetidine, ranitidine). However, due to logistical factors, short-course premedication with intravenously administered dexamethasone—given 30 min prior to paclitaxel infusion—has become customary in many centers.[1]
Author contributions
Conceptualization: Stefano D’Errico.
Investigation: Benedetta Baldari.
Methodology: Paola Frati, Vittorio Fineschi.
Supervision: Paola Frati, Vittorio Fineschi.
Writing – original draft: Stefano D’Errico, Benedetta Baldari.
Writing – review & editing: Mauro Arcangeli, Alessandro Santurro.
Footnotes
Abbreviations: C = complement, CARPA = C activation-related pseudoallergy, CrEL = Cremophor EL, FDA = Food and Drug Administration, HSR = hypersensitivity reactions, Med-RADAR = Medical Research on Adverse Drug Events and Reports.
How to cite this article: D’Errico S, Baldari B, Arcangeli M, Santurro A, Frati P, Fineschi V. Mast cells activation and high blood tryptase levels due to paclitaxel administration. Is Cremophor EL the culprit? A case report. Medicine. 2020;99:43(e22814).
Ethical approval and informed consent: This case dealt with a death of judicial interest, for which ethical approval and consent were requested and obtained from the Judicial Authority. Data processing complies with the general authorization for scientific research purposes granted by the Italian Data Protection Authority (March 1, 2012 as published in Italy's Official Journal no. 72 dated March 26, 2012) since the data do not entail any significant personalized impact on data subjects. Approval by an institutional and/or licensing committee is not required since experimental protocols are not applied in the study.
The authors received no funding.
The authors have no conflict of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the present study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
References
- [1].Kloover JS, den Bakker MA, Gelderblom H, et al. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer 2004;90:304–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shepherd GM. Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol 2003;24:253–62. [DOI] [PubMed] [Google Scholar]
- [3].Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003;21:194–200. [DOI] [PubMed] [Google Scholar]
- [4].Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol 1990;8:1263–8. [DOI] [PubMed] [Google Scholar]
- [5].Markman M, Kennedy A, Webster K, et al. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol 2000;18:102–5. [DOI] [PubMed] [Google Scholar]
- [6].Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol 2009;102:179–87. [DOI] [PubMed] [Google Scholar]
- [7].Irizarry LD, Luu TH, McKoy JM, et al. Cremophor EL-containing paclitaxel-induced anaphylaxis: a call to action. Community Oncol 2009;6:132–4. [PMC free article] [PubMed] [Google Scholar]
- [8].Bennett CL, Adegboro OS, Calhoun EA, et al. Beyond the black box: drug- and device-associated hypersensitivity events. Drug Healthc Patient Saf 2010;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williams C, Bryant A. Short versus long duration infusions of paclitaxel for any advanced adenocarcinoma. Cochrane Database Syst Rev 2011;5:CD003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hainsworth JD, Thompson DS, Greco FA. Paclitaxel by 1-hour infusion: an active drug in metastatic non-small-cell lung cancer. J Clin Oncol 1995;13:1609–14. [DOI] [PubMed] [Google Scholar]
- [11].Price KS, Castells MC. Taxol reactions. Allergy Asthma Proc 2002;23:205–8. [PubMed] [Google Scholar]
- [12].Feldweg AM, Lee CW, Matulonis UA, et al. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol 2005;96:824–9. [DOI] [PubMed] [Google Scholar]
- [13].Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol 2008;122:574–80. [DOI] [PubMed] [Google Scholar]
- [14].Trudeau ME, Eisenhauer EA, Higgins BP, et al. Docetaxel in patients with metastatic breast cancer: a phase II study of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol 1996;14:422–8. [DOI] [PubMed] [Google Scholar]
- [15].Kwon JS, Elit L, Finn M, et al. A comparison of two prophylactic regimens for hypersensitivity reactions to paclitaxel. Gynecol Oncol 2002;84:420–5. [DOI] [PubMed] [Google Scholar]
- [16].Rosenberg P, Andersson H, Boman K, et al. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol 2002;41:418–24. [DOI] [PubMed] [Google Scholar]
- [17].Peereboom DM, Donehower RC, Eisenhauer EA, et al. Successful re-treatment with taxol after major hypersensitivity reactions. J Clin Oncol 1993;11:885–90. [DOI] [PubMed] [Google Scholar]
- [18].O'Sullivan BT, Cutler DJ, Hunt GE, et al. Pharmacokinetics of dexamethasone and its relationship to dexamethasone suppression test outcome in depressed patients and healthy control subjects. Biol Psychiatry 1997;41:574–84. [DOI] [PubMed] [Google Scholar]
- [19].Koppler H, Heymanns J, Weide R. Dose reduction of steroid premedication for paclitaxel: no increase of hypersensitivity reactions. Onkologie 2001;24:283–5. [DOI] [PubMed] [Google Scholar]
- [20].Prieto Garcia A, Pineda de la Losa F. Immunoglobulin E-mediated severe anaphylaxis to Paclitaxel. J Investig Allergol Clin Immunol 2010;20:170–1. [PubMed] [Google Scholar]
- [21].Banerji A, Lax T, Guyer A, et al. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5 year review. J Allergy Clin Immunol Pract 2014;2:428–33. [DOI] [PubMed] [Google Scholar]
- [22].Tiotiu A, Clement-Duchene C, Vaillant P, et al. Anaphylactic reaction induced by paclitaxel in the treatment of non small cell lung cancer (NSCLC). Int J Clin Med Allergy 2013;1:1–2. [Google Scholar]
- [23].Szebeni J, Muggia FM, Alving CR. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer Inst 1998;90:300–6. [DOI] [PubMed] [Google Scholar]
- [24].Szebeni J, Alving CR, Savay S. Formation of complement-activating particles in aqueous solutions of taxol: possible role in hypersensitivity reactions. Int Immunopharmacol 2001;1:721–35. [DOI] [PubMed] [Google Scholar]
- [25].Dizon DS, Schwartz J, Rojan A, et al. Cross-sensitivity between paclitaxel and docetaxel in a women's cancers program. Gynecol Oncol 2006;100:149–51. [DOI] [PubMed] [Google Scholar]
- [26].Dye D, Watkins J. Suspected anaphylactic reaction to Cremophor EL. Br Med J 1980;280:1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gelderblom H, Verweij J, Nooter K, et al. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001;37:1590–8. [DOI] [PubMed] [Google Scholar]
- [28].Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794–803. [DOI] [PubMed] [Google Scholar]
- [29].Takashima S, Kiyoto S, Takahashi M, et al. Clinical experience with nanoparticle albumin-bound paclitaxel, a novel taxane anticancer agent, and management of adverse events in females with breast cancer. Oncol Lett 2015;9:1822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Picard M, Castells MC. Re-visiting hypersensitivity reactions to Taxanes: a comprehensive review. Clin Rev Allergy Immunol 2015;49:177–91. [DOI] [PubMed] [Google Scholar]
- [31].Essayan DM, Kagey-Sobotka A, Colarusso PJ, et al. Successful parenteral desensitization to paclitaxel. J Allergy Clin Immunol 1996;97:42–6. [DOI] [PubMed] [Google Scholar]
- [32].Moon C, Verschraegen CF, Bevers M, et al. Use of docetaxel (Taxotere) in patients with paclitaxel (Taxol) hypersensitivity. Anticancer Drugs 2000;11:565–8. [DOI] [PubMed] [Google Scholar]
- [33].Riezzo I, Bello S, Neri M, et al. Ceftriaxone intradermal test-related fatal anaphylactic shock: a medico-legal nightmare. Allergy 2010;65:130–1. [DOI] [PubMed] [Google Scholar]
- [34].D’Errico S, Frati P, Zanon M, et al. Cephalosporins’ cross-reactivity and the high degree of required knowledge. Case report and review of the literature. Antibiotics (Basel) 2020;9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Busardò FP, Frati P, Santurro A, et al. Errors and malpractice lawsuits in radiology: what the radiologist needs to know. Radiol Med 2015;120:779–84. [DOI] [PubMed] [Google Scholar]
- [36].La Russa R, Fineschi V, Di Sanzo M, et al. Personalized medicine and adverse drug reactions: the experience of an Italian teaching hospital. Curr Pharm Biotechnol 2017;18:274–81. [DOI] [PubMed] [Google Scholar]
- [37].Santurro A, Vullo AM, Borro M, et al. Personalized medicine applied to forensic sciences: new advances and perspectives for a tailored forensic approach. Curr Pharm Biotechnol 2017;18:263–73. [DOI] [PubMed] [Google Scholar]


