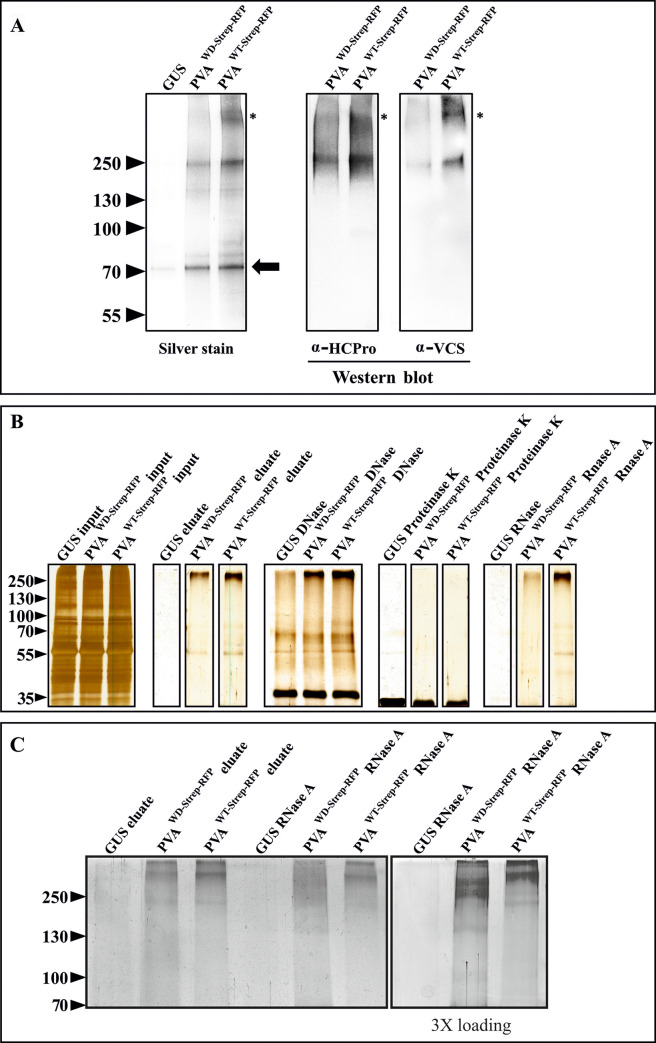
Fig 5. The WD mutation weakens VCS association with HCPro in HMW RNP complexes and the stability of the complexes.
(A) HCPro and VCS assemble into HMW complexes during infection. For affinity purification, PVAWD- Strep-RFP / PVAWT-Strep-RFP constructs were agroinfiltrated at OD600 = 0.1 and the infected local leaves were sampled at 3 dpi. Both HCPro and VCS antibodies recognized HMW bands with a similar electrophoretic mobility in the purified HCProWT-Strep-RFP samples. The uppermost HCPro and VCS containing band was missing in HCProWD-Strep-RFP samples (marked with asterisks) both in the silver-stained gel and in the western blots. Equal loading is demonstrated in the silver-stained gel by the low molecular weight bands present in the samples (marked with an arrow). All the samples were run in the same gel. (B) Validation of the HCProWD-Strep-RFP / HCProWT-Strep-RFP affinity purification procedure by SDS PAGE and silver staining. HMW complexes are visible in the HCProWD/WT-Strep-RFP eluates. The purified products were treated with DNase, Proteinase K and RNase A and subsequently subjected to SDS-PAGE. The silver-stained gels demonstrate the stability of the purified HMW complexes. (C) RNase degradation of HCPro-associated HMW RNP complexes. To achieve maximum separation of the HMW RNP complexes, samples similar to those in A) were loaded in varying quantities and run for a longer time in a 10% SDS-PAGE gel. In all the cases, PVAWD-Strep-RFP and PVAWT-Strep-RFP samples were loaded equally similarly as in A). Samples presented in the right panel are similar to those shown in the adjacent left panel except that three folds more was loaded to visualize the effect of RNase A treatment on complexes in PVAWD-Strep-RFP samples. All the samples in C) were part of the same gel but the right panel was developed for a longer time than the left panel during silver staining.