Graphical abstract
Abstract
The outbreak of a novel coronavirus (SARS-CoV-2) has caused a major public health concern across the globe. SARS-CoV-2 is the seventh coronavirus that is known to cause human disease. As of September 2020, SARS-CoV-2 has been reported in 213 countries and more than 31 million cases have been confirmed, with an estimated mortality rate of ∼3%. Unfortunately, a drug or vaccine is yet to be discovered to treat COVID-19. Thus, repurposing of existing cancer drugs will be a novel approach in treating COVID-19 patients. These drugs target viral replication cycle, viral entry and translocation to the nucleus. Some can enhance innate antiviral immune response as well. Hence this review focuses on comprehensive list of 22 drugs that work against COVID-19 infection. These drugs include fingolimod, colchicine, N4-hydroxycytidine, remdesivir, methylprednisone, oseltamivir, icatibant, perphanizine, viracept, emetine, homoharringtonine, aloxistatin, ribavirin, valrubicin, famotidine, almitrine, amprenavir, hesperidin, biorobin, cromolyn sodium, and antibodies- tocilzumab and sarilumab. Also, we provide a list of 31 drugs that are predicted to function against SARS-CoV-2 infection. In summary, we provide succinct overview of various therapeutic modalities. Among these 53 drugs, based on various clinical trials and literature, remdesivir, nelfinavir, methylpredinosolone, colchicine, famotidine and emetine may be used for COVID-19.
Significance
It is of utmost important priority to develop novel therapies for COVID-19. Since the effect of SARS-CoV-2 is so severe, slowing the spread of diseases will help the health care system, especially the number of visits to Intensive Care Unit (ICU) of any country. Several clinical trials are in works around the globe. Moreover, NCI developed a recent and robust response to COVID-19 pandemic. One of the NCI’s goals is to screen cancer related drugs for identification of new therapies for COVID-19. https://www.cancer.gov/news-events/cancer-currents-blog/2020/covid-19-cancer-nci-response?cid=eb_govdel.
1. Introduction
SARS-CoV-2 is a single-stranded enveloped RNA virus with a symmetrical nucleocapsid. SARS-CoV-2 targets cells via the viral structural spike (S) protein that binds to the angiotensin-converting enzyme 2 (ACE2) receptor to enter the host cell. Host type 2 transmembrane serine protease, (TMPRSS2) facilitates cell entry via the S protein [1]. Mechanistically, host proteases, especially TMPRSS2 cleave virus hemagglutinin to trigger internalization of the virus [2], [3]. Once inside the cell, viral polyproteins that encode the replicase-transcriptase complex are synthesized and the RNA-dependent RNA polymerase replicates viral RNA. Consequently, structural proteins are synthesized leading to completion of assembly and release of viral particles [4], [5], [6]. So far, promising drugs target different viral enzymes including 3-chymotrypsin-like protease, papain-like protease, and RNA-dependent RNA polymerase. In addition, multiple drugs target viral entry and immune regulation pathways such as suppressing the cytokine storm mediated by the virus [7], [8], [9]. COVID-19 pandemic is forcing the scientific community to develop novel therapies immediately, because no vaccine is available yet. Moreover, synthesis and evaluation of new drugs from preclinical to phase III trials is a time-consuming process. Several drugs that are being considered for COVID-19 therapy have already been used in cancer therapies. Similar to cancer cells, virus infected cells enhance the synthesis of nucleic acids, proteins and increase energy metabolism. Thus, drugs that are blocking specific cancer cell pathways may be effective in blocking viral replication as well. Hence, in this review we provide a comprehensive analysis of some cancer drugs and others that can be repurposed for the treatment of COVID-19.
2. SARS-CoV-2 primary mechanisms of cellular entry and infection
SARS-CoV-2 uses two major pathways to enter the cells. The virus can fuse with the plasma membrane to enter through the plasma membrane. The second route is by fusing with the endosomal membrane. Interestingly, coronavirus fusion depends on proteases in the virus local environment, which signifies the flexibility of coronavirus S proteins to respond to different signal proteins [10], [11]. Mechanistically, cellular entry of coronaviruses depends on the binding of the transmembrane spike (S) glycoprotein (forms homotrimers) [12] to a specific cellular receptor and subsequent S protein priming by cellular proteases. In doing so, SARS-CoV-2 recruits ACE2 as a receptor for cellular entry. Studies have shown that binding affinity of S protein and ACE2 is correlated with viral replication rate and disease severity [1], [13], [14]. Similarly, SARS-CoV-2 entry also depends on TMPRSS2 protease activity and cathepsin B/L activity.
When there are no exogenous or membrane-bound proteases available, coronaviruses can utilize clathrin or non-clathrin-mediated endocytosis for cellular internalization [15]. Surprisingly, for SARS-CoV-2, it is still not clearly understood where exactly fusion of viral and cellular membranes occur. It is possible that fusion occurs at the cell plasma membrane, and this has been proposed as the major cellular entry pathway [10]. Two main scenarios have been proposed for viral endocytosis. Viral RNA can enter the cytosol via the fusion pore as viral coat proteins do not directly enter the cell. Alternatively, the complex of SARS-CoV-2 and cell receptor (ACE2) undergoes endocytosis, and subsequently fusion of the viral membrane with the luminal face of the endosomal membrane promotes translocation of RNA into the cytosol [16], [17], [18].
3. Drug candidates being repurposed as potential therapeutics for COVID-19 treatment
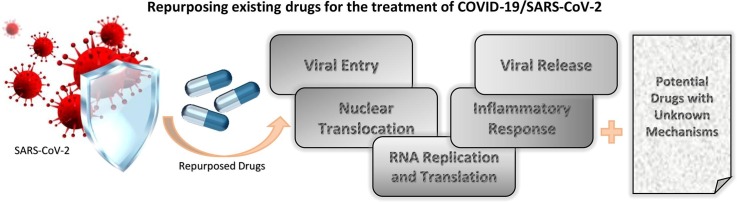
Although there are no approved specific drugs or vaccines that combat coronaviruses, there are several options that can theoretically fight the disease. These options include vaccines, monoclonal antibodies, oligonucleotide-based therapies, peptides, interferon therapies, and small-molecule drugs. Unfortunately, discovery of drugs that can have permanent cure for the disease may take several months to years. Based on crystallography data, some possibilities have been envisaged to control or prevent emerging infections of SARS-CoV-2. Protein structural data indicate that drug binding pockets in viral enzymes are conserved across SARS-CoV-2, SARS and MERS [19]. Thus, several investigators are trying to repurpose the existing drugs for MERS and SARS [20]. Although no effective drug treatments have been identified for COVID-19, several of them are being tested. These include drugs that are meant for cancers, HIV and malaria [20], [21]. Since COVID-19 is spreading uncontrollably across the globe, it is essential to discover new drugs, and it can be accomplished by repurposing drugs that are discussed in this article. In this review, first, we will discuss drugs that are currently being investigated for their use in treating patients. In the next section, we will focus on the drugs that are predicted to inhibit COVID-19 based on docking approaches using crystal structure of proteins of SARS-CoV-2. We identified 22 drugs (Fig. 1, Fig. 3 ) that are actively being pursued for testing/clinical trials. From these, we identified 6 drugs that are higly potent for COVID-19 infection (Fig. 1). Also we show another 31 drugs (Table 1 ) that are predicted to work against COVID-19 infection. The drugs that are currently considered for therapies against COVID-19 infection are targeted to different steps of viral infection including viral entry, translation, proteolysis, viral RNA replication, viral protein assembly and viral release.
Fig. 1.
List of drugs that are actively being considered for COVID-19 infection. The structures were collected from Kim et al [184].
Fig. 3.
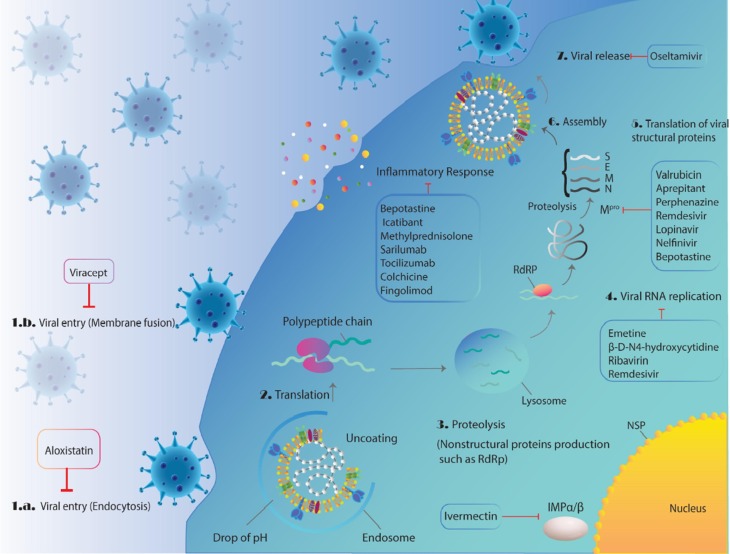
Schematic overview of the potential therapeutic drugs undergoing clinical trial or have been proposed against SARS-CoV-2 virus with their mechanism of action in different main steps of CoV life cycle in host cells including, viral endocytosis inhibitors, virus–cell membrane fusion inhibitors, the viral RNA-dependent RNA polymerase or replication inhibitors, viral protease inhibitors, and inflammatory response modulators.
Table 1.
Mechanism of action for the drugs that were identified based on structural data.
| Drug name | Mechanism/s | Ref |
|---|---|---|
| Ursolic acid | Ursolic acid (UA) is a pentacyclic triterpenoid carboxylic acid that has been reported to possess antioxidant and anti-tumor properties. These properties of UA have been attributed to its ability to suppress NF-κB activation to regulate the expression of inflammatory genes. | [185], [186] |
| Saikosaponin A | Saikosaponin A up-regulates LXRα expression and has shown potent anti-inflammatory activity. | [187], [188], [189] |
| Mulberroside A | Mulberroside A decreases the expressions of TNF-α, IL-1β, and IL-6 and inhibits the activation of NALP3, caspase-1, and NF-κB and the phosphorylation of ERK, JNK, and p38, exhibiting anti-inflammatory and antiapoptotic effects. | [190] |
| Troxerutin | Troxerutin, also known as vitamin P4, can inhibit the production of reactive oxygen species (ROS) and repress ER stress-mediated NOD activation. | [191], [192], [193] |
| Verbascoside | Verbascoside acts as an ATP-competitive inhibitor of PKC and has antitumor and anti-inflammatory activity. | [194] |
| Corosolic acid | Corosolic acid is a protein kinase C inhibitor and exhibits anti‐angiogenic and anti‐lymphangiogenic effects. | [195], [196] |
| cynaroside | Antioxidant and anti-inflammatory activity. | [197], [198] |
| Orientin | Orientin has shown anti-inflammatory, anti-oxidative and anti-tumor activity. | [199] |
| ε-Viniferin | ε-Viniferin displays a potent inhibitory effect on the CYP activities with potent antioxidant ability. | [200], [201] |
| Myricitrin | Antioxidant activity. | [202], [203] |
| Baicalin | Baicalin reduces the expression of NF-κB. Baicalin treatment inhibits the increased expression of the proinflammatory factors including TLR2/4, MyD88, p-NF-κB, and p- IκB, as well as increase the expression of IκB protein, an NF-κB inhibitor | [204], [205] |
| Corynoline | Corynoline is a reversible and noncompetitive acetylcholinesterase (AChE). Corynoline exhibits anti-inflammatory activity by activating Nrf2. | [206], [207] |
| Protostemonine | Protostemonine is an active alkaloid and has anti-inflammatory effects on asthma and gram-negative bacteria-induced acute lung injury. | [207], [208] |
| Amygdalin | Amygdalin has antitumor activity. Amygdalin inhibits NF-kβ and NLRP3 signaling pathways, and consequently has anti-inflammatory effect by reducing the expression of proinflammatory cytokines such as pro-IL-1β. | [209] |
| Paeoniflorin | Anti-inflammatory activity. | [210] |
| Taiwanhomoflavone A | Anti-inflammatory properties have been reported. Also, a SARS-CoV-2 MPro inhibitor with strong binding ability to other targets of SARS-CoV-2, like RdRp and hACE-2. | [203], [211] |
| Lactucopicrin 15-oxalate | Lactucopicrin has reported to be antimalarial compounds. Also, a SARS-CoV-2 MPro inhibitor with strong binding ability to other targets of SARS-CoV-2, like RdRp and hACE-2. | [203], [212] |
| Nympholide A | A SARS-CoV-2 MPro inhibitor with strong binding ability to other targets of SARS-CoV-2, like RdRp and hACE-2. | [203], [213] |
| Saquinavir | Saquinavir has been reported to inhibit invasion and angiogenesis via reducing of MMP expression and activity. Also, saquinavir could increase in MDR1 mediated drug-efflux to exert anti-HIV activity. | [214], [215], [216] |
| Phyllaemblicin B | Antiviral effects of Phyllaemblicin B are due to suppression of virus induced apoptosis. A SARS-CoV-2 MPro inhibitor with strong binding ability to other targets of SARS-CoV-2, like RdRp and hACE-2. | [203], [217] |
| Cassameridin | Moderate antifungal activity. | [203] |
| Chrysanthemin | Antitumor effects via apoptosis induction, caspase signaling pathway and loss of mitochondrial membrane potential. Also, a SARS-CoV-2 MPro inhibitor with strong binding ability to other targets of SARS-CoV-2, like RdRp and hACE-2. | [203], [218] |
| Scalarane | Anti-inflammatory role. | [219] |
| Astragaloside A | Antioxidant, anti-apoptotic and antivirus effects. | [220] |
| Ilexgenin A | Anti-inflammation and anti-angiogenesis effects through inhibition of STAT3 and PI3K pathways and suppressing the inflammatory cytokines including TNF-α and IL-6 levels. | [221] |
| Rutin | Rutin has shown to have antioxidant, anti-inflammatory, anti-allergic, anti-angiogenic and antiviral properties. | [222], [223], [224] |
| Glycyrrhizin (Glycyrrhizic acid) | Glycyrrhizic acid acting as a direct HMGB1 antagonist, with anti-tumor, anti-diabetic activities. | [225], [226], [227] |
| Dipsacoside B | Anti-inflammatory effects | [228] |
| Puerarin | Puerarin has been shown to be a 5-HT2C receptor antagonist. Puerarin also inhibits the expression of LPS-induced iNOS, COX-2 and CRP proteins through suppression of p65NF-κB nuclear translocation |
[229] |
| Morusin | Morusin acts as an antitumor, antioxidant, and anti-bacteria drug. Mechanistically, Morusin inhibits NF-κB and STAT3 activity. Morusin could also suppress breast cancer cell growth in vitro and in vivo through C/EBPβ and PPARγ mediated lipoapoptosis. In colorectal cancer, Morusin significantly inhibits the growth and clonogenicity of human colorectal cancer cells and suppressed the NF-κB activation. In addition, Morusin induced apoptosis in human prostate cancer cells by suppressing STAT3 activity. | [230], [231], [232] |
| Polyphyllin I | Polyphyllin I has been demonstrated to have strong anti-tumor activity in human non-small lung cancer cells. Polyphyllin I is an activator of the JNK signaling pathway and is an inhibitor of PDK1/Akt/mTOR signaling in human gastric carcinoma cells. Polyphyllin I induce autophagy, G2/M phase arrest and apoptosis in human glioma cells. | [233], [234], [235] |
3.1. Drug candidates that attack viral entry (membrane fusion endocytosis)
As shown in Fig. 3, SARS-CoV-2 enter the host cells by fusion and endocytosis. The following drugs potentially attack these steps.
3.1.1. Aloxistatin (E-64D)
Aloxistatin, is a cysteine protease inhibitor for calpains and cathepsins, and has an important regulatory role in neurodegeneration and cancer therapy. Cathepsin B and L could be considered potential biomarkers for cancer. Catalytic activity of these two proteases lead them to function as cell regulatory enzymes [22]. Moreover, calpain inhibition by aloxistatin has shown promising results in cataracts treatment. Aloxistatin inhibits hepatitis virus replication in a mouse model [23]. Mechanistically, this drug irreversibly forms a thioether bond thus modifying the active site of Cysteine. Nucleophilicity of the Cysteine’s active site helps aloxistatin to act selectively toward Cysteine proteases [22]. Aloxistatin inhibits lung cancer metastasis as well [24]. In addition, aloxistatin reduces cellular entry of SARS-CoV-2 by 92.3% since cathepsin L is a necessary factor for SARS-CoV-2 cell entry [25]. Further structural analysis indicated an interaction of membrane permeable aloxistatin with the active site of the SARS-CoV-2 main protease (Mpro), which is important in making nonstructural protein (NSP). Interestingly, it also binds to papain-like proteases with less specificity [26]. In summary, aloxistatin is a potential drug for COVID-19 that could potentially affect SARS-CoV-2 proteases.
3.2. Drug candidates that attack SARS-CoV-2 viral entry (membrane fusion)
3.2.1. Viracept (nelfinavir mesylate)
Viracept is an anti-retroviral drug that selectively inhibits human immunodeficiency virus (HIV) protease. Mechanistically, viracept prevents cleavage of gag-pol viral polyprotein that results in release of immature and noninfectious virions [27]. Previous results with SARS and MERS CoV have shown that Spike (S) glycoprotein is a major determinant of virus infectivity and immunogenicity [28]. Recent studies indicated the potential efficacy of viracept against SARS CoV-2 [29], [30]. Viracept inhibits S-n and S-o-mediated cell fusion resulted by SARS-CoV-2 Spike (S) glycoprotein [29], [30]. This evidence suggests that viracept blocks SARS-CoV-2 transfer and spread from cell-to-cell and making it more sensitive to neutralizing antibodies. Interestingly, viracept inhibits growth of cancer cells as well [31], [32].
3.3. Drug candidates that affect viral RNA replication and translation
Viral RNA is released into the cytoplasm, and translation of genomic RNA yields very large polypeptide, which undergoes proteolysis to generate RNA-dependent RNA polymerase (RDRP). Further, through the action of RNA polymerase, other proteins are made. Coronavirus has four structural proteins, including spike (S), membrane (M), envelope (E) and nucleocapsid (N). M is the most abundant structural protein. RNA replication and translation can be blocked by the drugs below.
3.3.1. β-D-N4-hydroxycytidine (NHC, EIDD-1931)
NHC is an orally bioavailable ribonucleoside analog with a broad-spectrum of antiviral activity against various unrelated RNA viruses. Cytidine analogs of NHC inhibits replication of Ebola virus (EBOV) [33]. Also, NHC blocks replication of hepatitis C virus (HCV) replicon [34]. Similarly, NHC inhibits human coronavirus [35], chikungunya virus [36], respiratory syncytial virus (RSV), hepatitis C virus, norovirus, influenza A (IAV) and B viruses, and Ebola virus [37]. Antiviral activity of NHC has been shown to be against human α-CoV HCoV-NL63, as well as β-CoV SARS-CoV [30], [32]. NHC is effective against Venezuelan equine encephalitis virus (VEEV) as well. Most VEEV virions released from NHC-treated cells showed mutated viral genomes, which cannot undergo replication and block the resistance to NHC [38]. In addition, NHC inhibits WT murine hepatitis virus (MHV) and MERS-CoV with minimal cytotoxicity [39]. Therapeutic administration of NHC improved pulmonary function, reduced virus titer and body weight loss in mice. Mechanistically, decreased MERS-CoV is associated with increased transition mutation frequency in viral but not host cell RNA resulting in lethal mutagenesis in CoV [39]. Altogether, these reports indicate that increased introduction of transition mutations in viral genomes after treatment with NHC, as well as a high genetic barrier to resistance, and these both characteristics of NHC might be helpful for COVID-19 treatment.
3.3.2. Remdesivir (GS-5734)
Remdesivir, is a nucleoside prodrug or nucleotide analog with potential efficacy against multiple viruses with sub micromolar range dose of administration [40], [41]. Nucleoside analogs (NAs) have been considered the most promising broad-spectrum anti-viral RNA-dependent RNA polymerase (RdRp) inhibitors and they have been effective in the treatment of multiple viral infections. Targeting viral replication within the host cell is one of the best anti-viral therapeutic approaches. These antiviral agents target viral replication enzymes. This could be due to their function as nucleoside analogs during viral replication that result in deadly mutations. Since many viruses depend on RdRp activity, remdesivir has good efficacy against a broad-spectrum of viruses including coronaviruses, SARS, MERS and CoVs [42], [43], [44]. In the case of SARS-CoV-2, it is shown that remdesivir inhibits viral replication, highlighting the importance of remdesivir for treatment of CoV infections [45].
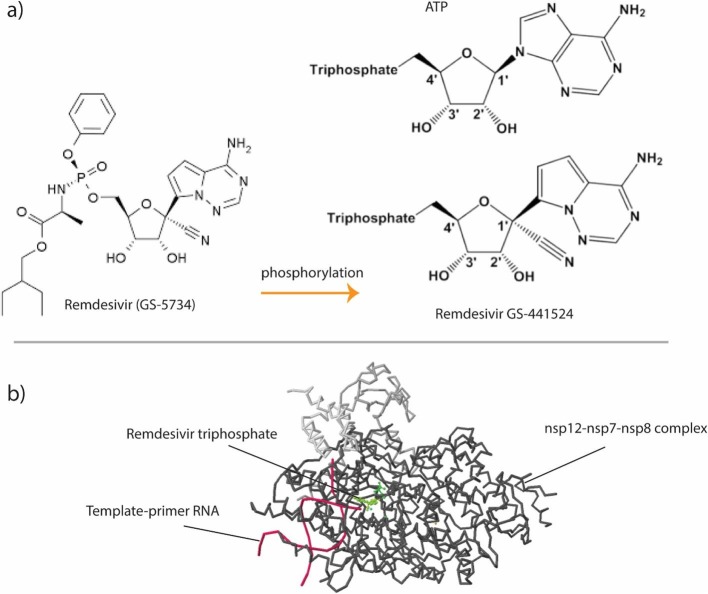
Mechanistically, remdesivir functions as an RdRp binding substrate that replaces ATP during polymerization (Fig. 2 ). Remdesivir (GS-5734) enters cells and metabolized into an adenosine nucleotide analog (GS-441524). Phosphorylation of GS-441524 will make a nucleoside triphosphate (NTP) to utilize as a substrate for RdRp [46]. Originally, it was shown that ATP could be the main substrate for SARS-CoV RdRp [47]. However, NTPs incorporation into viral replication machinery replaces ATP resulting in inefficient elongation, a process known as “chain termination” [42], [47]. In addition, NTPs do not show a significant inhibition for both human RNA Pol II and human mitochondrial RNA polymerases [48]. This possible mechanism of action has been detected for Nipah virus (NiV) RdRp as well. Despite potential efficacy of remdesivir, its application for CoV is challenging because of exonuclease activity of CoV nsp14 domain. This proofreading activity can remove the incorporated NTPs from viral RNA [49], [50], [51]. Three coronavirus (MERS-CoV, SARS-CoV, and SARS-COV-2) RdRp complexes have specific termination site at position i + 3, which means RNA synthesis is stopped after the addition of three more nucleotides, and this is often referred to as delayed chain-termination. However, higher concentration of natural nucleotide pool can dominate this RdRp-mediated termination. In addition, it is proposed that additional three nucleotides are necessary for providing protection against 3′-5′ exonuclease activity of nsp14 [52]. Studies on remedisivir recognition, incorporation and excision by nsp12 and nsp14 have provided key insights for new NA drug development [53]. Remdesivir is the first FDA approved drug for treatment of hospitalized 2019 coronavirus disease (COVID-19) patients. https://www.fda.gov/media/137564/download.
Fig. 2.
Remdesivir (GS-5734) modification (phosphorylation) into the adenosine nucleotide analog (GS-441524). Phosphorylation on GS-441524 will make a nucleoside triphosphate (NTP) that can be utilized as a substrate for RNA-dependent RNA polymerase (RdRp) (a). ATP could be the main substrate for NSP12 of SARS-CoV RdRp. NTPs incorporation into the viral replication machinery replaces ATP and finally results in inducing inefficient elongation (b).
Several clinical trials are being done worldwide to examine remedesivir function on COVID-19 patients. Gilead Sciences initiated two phase III clinical studies to evaluate efficacy of remdesivir on 1000 COVID-19 patients from all around the world. This study analyzes remdesivir efficacy and safety in two categories, a 5-day and a 10-day remedisivir regimen [54]. It was reported that compassionate use of remdesivir for patients with severe COVID-19 has 68% clinical improvement, however, 13% of patients died. Increased hepatic enzymes, diarrhea, rash, renal impairment, and hypotension were among the common adverse events in 60% of cases. Since this was done in a small cohort, more comprehensive studies must be considered to confirm clinical benefit of remdesivir in patients with severe COVID-19 [55]. A randomized, double-blind, placebo-controlled, multi-center trial from10 hospitals was done on 237 severe COVID-19 patients. The participants were assigned 2:1 ratio to receive either intravenous administration of remdesivir (200 mg of remdesivir on the first day followed by 100 mg per day during a 10-day medication) or the same conditions of placebo. Patients who received remdesivir showed a faster clinical improvement than the other group. Nevertheless, remdesivir treatment was stopped in 12% of cases because of the adverse results which were still less than the results of patients receiving placebo (12% vs 5%) [56]. Despite conventional antiviral drugs that have better efficacy at early stage of infection, remdesivir has been shown to be a potent drug in treatment of late stage COVID-19 patients [57].
3.3.3. Emetine
Emetine is traditionally used as an emetic and expectorant drug [58]. Its mechanism of action is through inhibition of ribosomal protein synthesis [58]. Emetine inhibits aminoacyl-sRNA transfer reaction, preventing its incorporation into polypeptide bonds [58]. A 2018 study reported that emetine inhibits Zika and Ebola virus infections by suppressing viral replication and subsequently decreasing viral entry [59]. Another study also showed that emetine and other alkaloids extracted from ipecac root are potent inhibitors of HIV reverse transcriptase (RT) [60]. Furthermore, emetine shown to have antiviral activity against human cytomegalovirus and rabies virus [61], [62] and has anticancer activity against various solid tumors [63]. Based on these viral inhibitory properties, emetine is now considered one of the drug candidates against COVID-19 infection. Interestingly, emetine inhibits SARS-CoV-2 replication in-vitro with EC50 of 0.46 μM and also has good synergistic effect with FDA approved remdesivir [64]. Thus, this drug will be a good candidate to repurpose for SARS-CoV-2 infection.
3.3.4. Ribavirin
Ribavirin (l-β-D-ribofuranosyl-l,2,4-triazole-3-carboxamide) is an antiviral agent. Ribavirin works against infection of several viruses including influenza virus, Lassa fever virus, Hantaan virus, and human immunodeficiency virus (HIV). Ribavirin received its FDA approval in 1986 as an aerosol for respiratory syncytial virus infected infants [65]. Also, ribavirin treatment is beneficial for acute leukemia patients [66]. Structurally, ribavirin is a guanosine nucleoside analog and resembles other purines including inosine and adenosine. Furthermore, ribavirin is therapeutically used in humans in combination with interferon-α in hepatitis C virus (HCV) infection. Ribavirin monophosphate interferes with viral RNA replication by inhibiting cellular protein inosine monophosphate dehydrogenase (IMPDH) [67]. IMPDH mediates inosine to GTP conversion and increases the expression of histone genes and E2F transcription factor, and thus controls cell proliferation [68]. Administration of ribavirin reduces intracellular GTP levels that disrupts RNA replication of virus [67], [68]. Viral RNA-dependent RNA polymerase can recruit ribavirin-triphosphate as a GTP analog or an ATP analog. However, ribavirin treatment in HCV weakly inhibits polymerase in vitro. Another possible antiviral mechanism for ribavirin is that it promotes lethal mutagenesis in viral RNA [67], [69]. A plethora of studies also proved that ribavirin can increase host cell immunity by inducing T-helper 1 (Th1) response (increased IFN-γ secretion). This response is through suppression of IL-10 secretion by T-reg cells [70], [71]. This drug has also been considered a combinational therapeutic with lopinavir/ritonavir and/or interferon-β1b against SARS-CoV-2 infections [72].
3.4. Drug candidates that only affect viral translation
3.4.1. Valrubicin
Valrubicin is an N-trifluoroacetyl 14-valerate derivative of anthracycline doxorubicin, and known to have anti-tumor activity. However, valrubicin is less effective compared to doxorubicin [73] in human bladder cells. Valrubicin is an anthracycline chemotherapy drug and its mechanism of action is histone loss, thus disruption of DNA repair process in tumor cells [73]. Valrubicin intercalates with dsDNA [74], [75]. Valrubicin mediates formation of DNA cleavable complexes and inhibit topoisomerase II activity leading to defects in DNA replication and transcription. Valrubicin has antiangiogenic function by binding to proteasome complex [76]. In addition, valrubicin mediates tumor cell apoptosis through binding to PKC and regulating downstream pathways [77]. Valrubicin can bind to and inhibit SARS-CoV-2 Mpro. and proteases [78], [79]. Thus, valrubicin is a good candidate drug for COVID-19.
3.4.2. Perphenazine
Perphenazine (PPZ) is an antipsychotic drug classified as a piperazinyl phenothiazine. Phenothiazines, including PPZ, suppress T cell acute lymphoblastic leukemia [80]. Perphenazine directly binds to protein phosphatase 2A, a well-conserved serine/threonine phosphatase, and induces dephosphorylation of ERK1/2 and Akt that in turn trigger apoptosis. So far, there are two clinical trials reported on this drug. First, a phase IV trial, investigated the effectiveness of PPZ (in combination with other antipsychotic drugs) in children and adolescents who have gained weight on their antipsychotic medications [NCT00806234]. Second trial investigated safety and efficacy of PPZ on schizophrenia patients [NCT00802100]. Although PPZ is popularly known as an antipsychotic drug, currently, it is also being investigated for possible antiviral effect against COVID-19. PPZ is one of the compounds predicted to be able to bind to the amino acid pocket site of 2019-nCoV and interferes its function [81]. Thus, this drug is a good candidate drug for COVID-19 infection.
3.4.3. Homoharringtonine
Homoharringtonine (HHT) is one of the alkaloid compounds extracted from Cephalotaxus harringtonia plant [82]. Also another product extracted from Cephalotaxus fortunei plant, a related species of Cephalotaxus harringtonia, is widely used in Chinese folk medicine for its antiparasitic, anti-inflammatory and antineoplastic effects [83]. HHT and harringtonine are the main alkaloids isolated from Cephalotaxus harringtonia. HHT can be distinguished from harringtonine due to the presence of methylene group on its side chain [83]. Though both alkaloids have similar biological and therapeutic potential, HHT has better extraction yield [83], [84]. HHT has a positive effect in treatment of chronic myelogenous leukemia (CML), acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) [85], [86]. Mechanistically, HHT inhibits translation of proteins through binding and interacting with the A-site in the peptidyl transferase of the ribosome [83], [87], [88]. HHT inhibits SARS-CoV-2 replication in vitro and thus the drug is being investigated for potential therapeutic function for COVID-19 infection [89].
3.5. Drug candidates that affect COVID-19 related inflammatory response
It is known that SARS-CoV-2 infects airway alveolar epithelial, vascular endothelial cells, and macrophages. SARS-CoV-2 infection results in aggressive inflammation and this is due to increased secretion of several interleukins and interferons. The below described drugs inhibit various inflammatory responses.
3.5.1. Fingolimod
Fingolimod (FTY720, Gilenya), is a sphingosine analog and a sphingosine 1-phosphate (S1P) receptor modulator. Fingolimod is the first FDA-approved drug for multiple sclerosis (MM)- a CNS related disease results in oligodendrocyte (OLG) loss. Fingolimod has been shown to be an effective drug for various cancers [90]. Fingolimod is phosphorylated by sphingosine kinase 1 and 2 (SphK1 and SphK2), and binds as an analog of S1P to the G-protein coupled S1P receptor subtypes S1P1, S1P3, S1P4, and S1P5 [91], [92]. Fingolimod showed decreased antiviral T-cell response against varicella-zoster virus (VZV) in multiple sclerosis patients [93]. Also, fingolimod is considered a potential therapeutic drug against SARS-CoV-2. Coronaviruses can spread into the CNS and fingolimod is capable of preventing T-cell penetration into the CNS and regulates blood–brain barrier (BBB) permeability. However, some reports show that there is no correlation between SARS-CoV-2 infection and lymphocyte levels in fingolimod treated cases [94], [95]. Thus, this drug needs a further evaluation for COVID-19 patients.
3.5.2. Colchicine
Colchicine, a natural product extracted from a plant named genus Colchicum (autumn crocus), has been used for inflammatoy arthritis and gout for centuries [96]. Colchicine disrupts many inflammation related cellular pocesses including inflammosome activation, inflammatory cell chemotaxis, leukotrienes production, cytokine production and phagocytosis. Colchicine inhibits polymerization of tubulin and microtubule assembly. Since these pathways are involved in many inflammatory diseases, colchicine could be a potential therapeutic candidate for further investigation [97]. Colchicine inhibits tumor growth by inducing apoptosis [98]. The NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome is an intracellular sensor capable of recognizing a plethora of pathogenic microbial motifs. It can trigger an inflammatory type of cell death through caspase-1 activation and its related secretion of pro-inflammatory cytokines, IL-1β and IL-18 [99], [100]. In addition, NLRP3 causes cellular stress, which is a part of immune response in autoimmune disorders, neurodegenerative diseases and tumor development. NLRP3 is expressed in immune cells specially antigen presenting cells (APCs) such as macrophages, and is upregulated through TLR-induced NF-κB activation [101]. Recent studies indicate that colchicine is a potential drug for anti-inflammatory response via NLRP3 inflammasome and cytokine storm suppression in COVID-19 patients [102], [103].
3.5.3. Tocilizumab
Tocilizumab is a monoclonal antibody that has many therapeutic functions. Interleukin-6 (IL-6) is a pleiotropic cytokine with known multiple functions such as inflammation, and oncogenesis. Binding of IL-6 to IL-6 receptor (IL-6R) induces homodimerization and recruitment of glycoprotein 130 (gp130), which leads to activation of downstream signaling. Emerging evidence suggests that high levels of IL-6 are correlated with poor prognosis in breast cancer patients. IL-6 appears to play a critical role in growth and metastasis of breast cancer cells, renewal of breast cancer stem cells (BCSCs), and drug resistance of BCSCs, making anti-IL-6/IL-6R/gp130 therapies promising options for treatment and prevention of breast cancers [104]. Recent reports showed anticancer effects of tocilizumab in a colon cancer xenograft model and effects of combination therapy of tocilizumab and interferon-alpha against renal cell carcinoma [105]. Tocilizumab also inhibited tumor growth of trastuzumab resistant breast cancer cells [106]. Cytokine storms mediated by overproduction of proinflammatory cytokines have been observed in a large population of critically ill patients infected with COVID‐19. [107], [108], [109]. The most recent data from China suggested that IL‐6 is one of the most important cytokines involved in COVID‐19‐induced cytokine storms (REF). IL-6 has been shown to regulate immune response, inflammation and oncogenesis. Mechanistically, IL-6 binds to IL-6R (IL-6 receptor) and gp130 to induce homodimerization of gp130. This homodimerization phosphorylates tyrosine residues (Tyr759) in the cytoplasmic domain of gp130. Activated gp130 induces phosphorylation of JAK1, JAK2 and Tyk2 result in activation of STAT1 and STAT3. This JAK- mediated phosphorylation of tyrosine residues in gp130 activates two downstream signaling pathways including JAK/STAT and ERK/MAPK. Consistent with this, soluble form of IL-6R (sIL-6R) also binds to IL-6R and forms a receptor complex with gp130 to further activate these signaling pathways [110]. IL-6 also plays a key role in T helper cell differentiation by regulating balance between IL-17 (T helper 17 cells) and regulatory T-cells (T-reg) [111]. Tocilizumab (TCZ) is an anti-human recombinant monoclonal antibody belongs to the immunoglobulin G1K subclass that binds to both soluble and membrane-bound interleukin 6 receptors (IL-6R) [112]. Tocilizumab has been approved by the FDA for rheumatoid arthritis (RA). In addition, tocilizumab has been shown to be a potential option for other inflammatory diseases, such as Castleman’s disease [113]. Since tocilizumab is an antibody for IL-6 receptor and potential alleviator of inflammatory response, this drug has been tried in COVID-19 patients and observed improved clinical outcome [114].
3.5.4. Sarilumab
Sarilumab, a human anti–IL-6R monoclonal antibody that has been used for rheumatoid arthritis and ankylosing spondylitis [113]. Similar to tocilizumab, sarilumab blocks IL-6-induced inflammatory signaling cascade by binding to both IL-6R and sIL-6R [115]. Also, sarilumab inhibits growth of xenograft tumors of DU145 (prostate), Calu3 (lung), and A549 (lung) either as a single agent or in combination with VEGF blocker aflibercept [116]. Sarilumab, like tocilizumab, binds specifically with high affinity to a unique epitope on IL6R (both membrane-bound and soluble IL-6R), and effectively blocks both cis- and transactivation of IL-6 signaling. Regeneron, a pharmaceutical company started a clinical trial of sarilumab on COVID-19 patients and the final data yet to be published.
3.5.5. Methylprednisolone
Methylprednisolone and its derivatives, methylprednisolone acetate succinate, and methylprednisolone sodium, are synthetic glucocorticoids used mainly as anti-inflammatory or immunosuppressive agents. Methylprednisolone has been suggested for palliative therapy for terminal cancer patients and it is five times more potent in its anti-inflammatory activity compared to hydrocortisone (cortisol) [117]. Methylprednisolone’s mechanism of action is through its binding to intracellular glucocorticoid receptor. Upon binding, this complex translocates to the nucleus, where it interacts with specific DNA sequences, resulting in suppression of downstream target genes. The methylprednisolone-glucocorticoid receptor complex binds to and blocks promoter sites of proinflammatory genes [118]. This leads to inhibition of synthesis of inflammatory cytokines by blocking the function of transcription factors such as nuclear factor-kappa-B (NF-kB) [119]. Presently, methylprednisolone is being tested in a number of COVID-19 related clinical investigations. A retrospective cohort study of 201 COVID-19 patients (median age-51 years), who developed acute respiratory distress syndrome, indicated that treatment with methylprednisolone decreased mortality [120]. Furthermore, methylprednisolone treatment resulted in good recovery of COVID-19 post-transplant patients with compromised immune systems [121], [122].
3.5.6. Icatibant (HOE-140, JE-049)
Icatibant is a highly specific competitive antagonist for the bradykinin type 2 (B2) receptor. It is an analog of bradykinin and is composed of 10 amino acids, five of which are synthetic and resistant to degradation of ACE2. These amino acids are needed to inactivate Des-Arg9-bradykinin (DBK), the potent ligand of bradykinin receptor type 1 (B1) [123]. The problem of pulmonary edema of COVID-19 patients could be due to activation of B1 and B2 receptors in lungs. ACE2 inactivates the ligands of B1 and thus, lung environment is prone for local vascular leakage leading to angioedema [124]. In vitro studies have confirmed that icatibant is a highly selective competitive antagonist for the B2 receptor [125]. In addition, icatibant blocks vascular functions of bradykinin in vivo [126]. Bradykinin plays a major role in developing pulmonary edema in COVID-19 patients due to inflammation caused by Des-Arg(9)-bradykinin. Thus, it is suggested that icatibant might be helpful in suppressing pulmonary edema and, thereby, will improve the clinical outcomes in COVID-19 patients [127], [128], [129].
3.6. Drug candidates that affect viral release
Viral assembly occurs in vesicles and the virus is exocytosed by fusion of virus containing vesicles with the plasma membrane and Ostelmivir blocks this step.
3.6.1. Oseltamivir
Oseltamivir is an ethyl ester oral prodrug against neuraminidase (an enzyme which is expressed on the viral surface) of influenza A and B virus. This drug is approved for treatment and prophylaxis of influenza A and B [130]. Once it enters cells, oseltamivir converts into an active form (GS4071) [130]. Mechanistically, lipophilic side chain on GS4071 binds to the hydrophobic pocket of the active site of the viral neuraminidase. As a result, it impairs the ability of neuraminidase to cleave sialic acid residues on the surface of the infected host cells and subsequently inhibits release of progeny virions from the infected cells. Inhibition of virions suppresses viral movement within the respiratory tract [130]. The substrate binding domain of influenza neuraminidase is highly conserved due to its critical role in viral replication. However, it is possible that resistant virus may be developed, and the resistance could be due to mutations in the viral neuraminidase or hemagglutinin or both. For example, clinical isolates from treated patients have been shown to be resistant [130], [131], [132], [133]. Surprisingly, oseltamivir enhances breast tumor growth [134]. Recently, oseltamivir was administered for COVID-19 in china, either with or without antibiotics and corticosteroids combination [135]. In addition, oseltamivir is also used in a clinical trial with several combinations with chloroquine and favipiravir [136].
4. Other drug candidates that are known to have good therapeutic effect against SARS-CoV-2 infection, but their mechanisms of action are unknown
4.1. Famotidine
It is a competitive antagonist for histamine H2-receptor. Famotidine acts as an inhibitor for gastric secretion. The preventive effect of famotidine on gastric lesions is attributable not only to suppression of acid secretion but to activation of gastric mucosal defensive mechanisms [137], [138]. Orally delivered famotidine has shown improved outcomes in COVID-19 patients [139], [140].
4.2. Almitrine
This drug is a peripheral chemoreceptor agonist, inhibits selectively Ca2 + -dependent K + channel. In vivo data showed that Almitrine could enhance hypoxic pulmonary vasoconstriction and enhanced overall ventilation/perfusion ratio [141]. It has been hypothesized that intravenous almitrine will reduce the need for ventilators patients with COVID-19 related pneumonia https://clinicaltrials.gov/ct2/show/NCT04357457.
4.3. Amprenavir
This drug has been shown to have an HIV protease inhibitory effect and is used against HIV infection. In addition, amprenavir suppressed hepatocarcinoma cell growth in vivo through inhibition of angiogenesis [142], [143]. Amprenavir forms an inhibitor-enzyme complex with HIV protease preventing the normal maturation process of HIV and formation of mature infectious virions. Amprenavir inhibits both HIV-1 and HIV-2 in vitro; however, the FDA approval is only against HIV-1 [144]. Unpublished reports based on bioinformatics analysis indicate that amprenavir may be a good drug for treatment of COVID-19
4.4. Cromolyn sodium
Cromolyn sodium functions as a GSK-3β inhibitor. Cromolyn sodium inhibits release of initiators of inflammation, mediated by specific antigens from mast cells. Cromolyn sodium may also inhibit the activity of other cell types that produce inflammation [145], [146], [147]. Similar to cancer cells, SARS-CoV-2 activates NFκB pathway and inhibitors of NFκB reduces inflammation associated with viruses. Cromolyn sodium inhibits NFκB in pancreatic cancer patients [148] and inhibits inflammation in several diseases [149]. Thus, cromolyn sodium may be effective in decreasing inflammation and cytokine storm in COVID-19 patients.
4.5. Hesperidin
This drug functions as an anti-inflammatory drug. For instance, hesperidin is shown to reduce inflammation as well as inflammatory pain via reduction of cytokine production, NF-κB activity, and oxidative stress. Anticancer effects of hesperidin are associated with its antioxidant and anti-inflammatory activities. Hesperidin inhibits tumor cell metastasis, angiogenesis, and chemoresistance [150], [151]. Unpublished molecular docking studies indicate that hesperidin may bind to multiple regions of SARS-CoV-2 (spike protein, ACE2 and proteases) https://www.drkarafitzgerald.com/2020/04/17/8-potential-natural-anti-avoid-compounds/, and thus hesperidin is a potential drug of COVID-19 therapies. Similar to hesperidin, Biorobin binds to multiple regions of SARS-CoV-2 [152], and hence suggested to be a good drug for COVID-19.
5. Discussion
COVID-19 pandemic is an untimely global healthcare challenge that requires timely social, environmental, and therapeutic interventions to halt or minimize the growing number of casualties. Globally, there are more than 31 million documented cases and approximately 960,000 reported deaths from COVID-19 as of September 2020. While these numbers are steadily increasing, there remain a few established standards of care and therapeutic options available. To date, there is still no available vaccine, although there are several options in various clinical trial stages. To effectively combat the rapid spread of SARS-CoV-2 infection and transmission between people, urgent clinical and therapeutic intervention must be put in place. One can search for a potent and reliable therapy for COVID-19 using the knowledge of drugs that are being used to treat other diseases. Herein, we have described mechanisms of action of leading antiviral, anticancer, and other relevant drugs that are currently being repurposed for the treatment of SARS-CoV-2 infection and COVID-19. In addition, we also briefly described drugs that have potential anti-SARS-CoV-2 effects, but their mechanisms of action are yet to be elucidated. The second category of drugs were identified using docking strategies based on crystal structure of SARS-CoV-2 proteins and potential binding pockets of drugs on these proteins. In this review, we focused on describing drug candidates that are presently being repurposed and how these therapeutic compounds can inhibit SARS-CoV-2 cellular entry, replication, translation of structural proteins and viral release.
Host cell entry is the first step in the life cycle of SARS-CoV-2 [153], and thus, this process constitutes a target for therapeutic inhibition. SARS-CoV-2 cellular entry occurs either through endocytosis or through fusion with host cell receptors [15]. Viral entry is an important determinant of coronavirus infection and COVID-19 pathogenesis. Therefore, drug candidates with the potential to inhibit SARS-CoV-2 entry into cells are in urgent demand. Here, we report that arbidol and viracept are drug candidates that have the potential to inhibit SARS-CoV-2 entry through membrane fusion while aloxistatin, chloroquine and hydroxychloroquine (HCQ) are capable of inhibiting viral entry through endocytosis [154], [155], [156]. Research studies have reported puzzling and sometimes controversial findings on the mechanisms by which SARS-CoV-2 uses for entry into cells. It has been shown that entry of SARS-CoV-2 into lung cells occur by fusion at the plasma membrane [15], whereas entry into non-lung cells such as Vero E6 cells occurs through spike mediated fusion of viral membranes with endosome membranes [1]. Additional studies have reported that HCQ does not inhibit SARS-CoV-2 infection of lung cells [157], despite it being a robust inhibitor of SARS-CoV-2 entry into Vero E6 cells which typically occurs via the endosome route [158]. Therefore, this implies that HCQ might have a cell-specific effect. In conclusion, more research studies will have to be conducted on HCQ before its clinical use can be encouraged.
Another molecular mechanism that enhances SARS-CoV-2 infection process is viral replication. The replication of the viral genome within the infected cells is a critical phase of the SARS-CoV-2 life cycle [159]. Thus, this process of viral replication constitutes another target for the inhibition of COVID-19. Viral replication event is a complex process involving the synthesis of both genomic and multiple sub-genomic RNA species, and the assembly of progeny virions by a pathway that is unique for this enveloped RNA virus [5]. The replication process involves the action of viral and host proteins in order to perform RNA polymerization, proofreading and final capping [160]. One of the widely studied drug candidates that is capable of inhibiting SARS-CoV- 2 replication in host cells is remdesivir [64]. In addition to remdesivir, emetine, ribavirin, and β-D-N4-hydroxycytidine are also among the drug candidates, that are capable of inhibiting SARS-CoV-2 replication inside target cells [161].
It should be noted that screening of most approved drug candidates was performed using workhorse cell line: Vero E6 [1], [162], [163]. Therefore, caution must be used when interpreting relevance of the data sets derived from studies that tested the efficacy of drugs based on data coming from just one cell line. It is known that different cells often respond differently to drug treatments [164], and thus testing these anti-SARS-CoV-2 drugs on multiple cell lines, especially lung cells, will largely help in identifying a more reliable set of drugs for treating COVID-19 patients. In order for these drugs to advance to the clinical trial stage, cell specificity, dosing, half-life, route of administration and half maximal inhibitory concentration (IC50) are critical components that need to be properly investigated to circumvent undesired patient outcomes [165]. For example, if the IC50 of any of these drugs in vitro is greater than what can be achieved with oral dosing, then the drug will never prove useful for COVID-19 patients. Thus, it is essential to look into all these parameters before a drug can be recommendd for clinical use.
Other cellular and molecular processes that constitute potential targets for therapeutic prevention of SARS-CoV-2 infection are nuclear translocation of virus, translation of structural proteins and viral release [166]. One of the symptoms of COVID-19 is the induction of inflammatory responses which triggers the release of cytokines especially IL-6 [167]. Some of the drugs we listed here have capability to reduce inflammatory side-effects of SARS-CoV-2 infection. While we have presented a list of potential antiviral drug candidates, we however think that only a limited number of them will prove clinically relevant for treatment and prevention of SARS-CoV-2 infection. Remdesivir has been shown as a promising drug. Clinical improvement was seen in 68% of the hospitalized patients for severe Covid-19 who were treated with remdesivir [55]. In addition, remdesivir is also effective in shortening the time to recovery and decreasing respiratory tract infection in adult patients hospitalized with Covid-19 [168]. So far, administration of chloroquine and hydroxychloroquine have been controversial as discussed before. The outcomes of recent clinical trials of chloroquine have yielded new information for its usage on COVID-19 patients. Adjunctive therapy for several acute SARS-CoV-2 infected patients showed that higher dosage of chloroquine is lethal and is not recommended especially when combined with azithromycin and oseltamivir. However, lower dosage showed enough safety and efficacy to treat these patients [169], [170], [171], [172]. HCQ has been shown to be less toxic compared to CQ, and thus it is a reasonable treatment option [173]. However, several studies showed the controversial usage of HCQ for COVID-19. It was suggested that different factors such as the stage of the disease, treatment duration and combination therapy, must be taken into consideration to obtain an efficient clinical outcome with HCQ [174], [175]. Methylprednisonine is another drug of interest. Early administration of prolonged methylprednisolone treatment is correlated with a significantly lower possibility of death (71%) and decreased ventilator dependence in patients with severe COVID-19 pneumonia. Consistent with this, another study indicated that single-dose methylprednisolone has no obvious negative impact on viral removal and production of specific IgG. However, it is effective in stopping the inflammatory cascade [176], [177]. Although early, low-dose and short-term administration of methylprednisolone is associated with better clinical outcomes, more randomized controlled trials are needed to confirm these findings [178]. Another potential drug, colchicine is shown a clinical improvement by reducing the length of hospitalization and dependency on supplemental oxygen therapy [179], [180]. Famotidine is correlated with lower hazard of mortality [181]. Although famotidine reduced the rates of mortality of the COVID-19 patients, there is no direct evidence of inhibitory effects of famotidine on SARS-CoV-2 replication [182]. Hence, further investigation of its molecular mechanism of action might be important in the context of COVID-19. Emetine is another drug which is effective against SARS-CoV-2 virus in Vero E6 cells. Interestingly, combination therapy of remdesivir and emetine shown to exert synergistic effects against the SARS-CoV-2 virus [64]. Furthermore, nelfinavir, an HIV-1 protease inhibitor, potently inhibits replication of SARS-CoV-2 [183]. Altogether, Remdesivir, Nelfinavir, Methylprednisolone, Colchicine, Famotidine and emetine are potentially considered for use in clinical trial applications or be recommended for further confirmatory studies.
Overall, this review will serve as a very timely and useful compilation of drugs being studied and repurposed for the treatment and prevention of SARS-CoV2 infection. Importantly, the mechanisms of action of these drugs as described herein will serve as a necessary guide for future studies investigating other COVID-19 therapeutic modalities and interventions.
Acknowledgments
Acknowledgments
The authors would like to thank the Alahari lab for their constructive criticism and positive feedbacks during the drafting and writing of this manuscript. All authors wish to thank all research groups around the world who are conducting biomedical, clinical and translational studies, investigating potent and effective SARS-CoV-2/COVID-19 therapeutic drug candidates.
Authorship contributions
H.Y, L.M, and S.C.O wrote and prepared the manuscript draft. N.A. Worked on couple of sections. Finally, S.A revised the final draft.
Footnotes
The authors would like to thank Fred G. Brazda foundation and LSU School of Medicine for financial assistance.
References
- 1.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böttcher E., Matrosovich T., Beyerle M., Klenk H.-D., Garten W., Matrosovich M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 2006;80(19):9896–9898. doi: 10.1128/JVI.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? AACR. 2020 doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehr A.R., Perlman S. Springer; 2015. Coronaviruses: An Overview of their Replication and Pathogenesis, Coronaviruses; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet. Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5(1) doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 10.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers as a potential target for antiviral development. Antiviral Res. 2020;104792 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.M. Hoffmann, H. Kleine-Weber, N. Krüger, M.A. Mueller, C. Drosten, S. Pöhlmann, The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells, BioRxiv (2020).
- 12.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. Elsevier. 2019:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang N., Shen H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020;16(10):1724. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glebov O.O. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J. 2020 doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A. Bayati, R. Kumar, V. Francis, P.S. McPherson, SARS-CoV-2 uses clathrin-mediated endocytosis to gain access into cells, bioRxiv (2020).
- 19.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 20.Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P., Huang X., Peiris M., Yen H.L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siklos M., BenAissa M., Thatcher G.R. Cysteine proteases as therapeutic targets: does selectivity matter? A systematic review of calpain and cathepsin inhibitors. J. Acta Pharm. Sin. B. 2015;5(6):506–519. doi: 10.1016/j.apsb.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto H.-H., Schirmeister T. Cysteine proteases and their inhibitors. J. Chem. Rev. 1997;97(1):133–172. doi: 10.1021/cr950025u. [DOI] [PubMed] [Google Scholar]
- 24.Kundu S.T., Grzeskowiak C.L., Fradette J.J., Gibson L.A., Rodriguez L.B., Creighton C.J., Scott K.L., Gibbons D.L. TMEM106B drives lung cancer metastasis by inducing TFEB-dependent lysosome synthesis and secretion of cathepsins. Nat. Commun. 2018;9(1):2731. doi: 10.1038/s41467-018-05013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiser K., McLean P.F., Davis C.T., Fogelson B., Gordon H.B., Jacobson P., Hurst B.L., Miller B.J., Alfa R.W., Earnshaw B.A. Identification of potential treatments for COVID-19 through artificial intelligence-enabled phenomic analysis of human cells infected with SARS-CoV-2. J. bioRxiv. 2020 [Google Scholar]
- 26.L.L. Palese, The structural landscape of SARS-CoV-2 main protease: hints for inhibitor search, (2020).
- 27.Pai V.B., Nahata M.C. Nelfinavir mesylate: a protease inhibitor. Ann. Pharmacother. 1999;33(3):325–339. doi: 10.1345/aph.18089. [DOI] [PubMed] [Google Scholar]
- 28.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.F. Musarrat, V. Chouljenko, R. Nabi, A. Dahai, S. Jois, K. Kousoulas, The anti-HIV Drug Nelfinavir Mesylate (Viracept) is a Potent Inhibitor of Cell Fusion Caused by the SARS-CoV-2 Spike (S) Glycoprotein Warranting further Evaluation as an Antiviral against COVID-19 infections, bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 30.M. Ko, S.Y. Chang, S.Y. Byun, I. Choi, A.L.P.H. d'Alexandry, D. Shum, J.-Y. Min, M.P. Windisch, Screening of FDA-approved drugs using a MERS-CoV clinical isolate from South Korea identifies potential therapeutic options for COVID-19, bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 31.Gills J.J., Lopiccolo J., Dennis P.A. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008;4(1):107–109. doi: 10.4161/auto.5224. [DOI] [PubMed] [Google Scholar]
- 32.Gills J.J., Lopiccolo J., Tsurutani J., Shoemaker R.H., Best C.J., Abu-Asab M.S., Borojerdi J., Warfel N.A., Gardner E.R., Danish M., Hollander M.C., Kawabata S., Tsokos M., Figg W.D., Steeg P.S., Dennis P.A., Nelfinavir A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin. Cancer Res. 2007;13(17):5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 33.Reynard O., Nguyen X.-N., Alazard-Dany N., Barateau V., Cimarelli A., Volchkov V.E. Identification of a new ribonucleoside inhibitor of Ebola virus replication. Viruses. 2015;7(12):6233–6240. doi: 10.3390/v7122934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costantini V.P., Whitaker T., Barclay L., Lee D., McBrayer T.R., Schinazi R.F., Vinjé J. Antiviral activity of nucleoside analogues against norovirus. Antiviral Ther. 2012;17(6):981. doi: 10.3851/IMP2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pyrc K., Bosch B.J., Berkhout B., Jebbink M.F., Dijkman R., Rottier P., van der Hoek L. Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob. Agents Chemother. 2006;50(6):2000–2008. doi: 10.1128/AAC.01598-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehteshami M., Tao S., Zandi K., Hsiao H.-M., Jiang Y., Hammond E., Amblard F., Russell O.O., Merits A., Schinazi R.F. Characterization of β-d-N4-hydroxycytidine as a novel inhibitor of chikungunya virus. Antimicrob. Agents Chemother. 2017;61(4):e02395–e2416. doi: 10.1128/AAC.02395-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toots M., Yoon J.-J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11(515) doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urakova N., Kuznetsova V., Crossman D.K., Sokratian A., Guthrie D.B., Kolykhalov A.A., Lockwood M.A., Natchus M.G., Crowley M.R., Painter G.R. β-D-N4-Hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J. Virol. 2018;92(3):e01965–e2017. doi: 10.1128/JVI.01965-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.T.P. Sheahan, A.C. Sims, S. Zhou, R.L. Graham, A.J. Pruijssers, M.L. Agostini, S.R. Leist, A. Schäfer, K.H. Dinnon, L.J. Stevens, An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Science translational medicine (2020). [DOI] [PMC free article] [PubMed]
- 40.D. Siegel, H.C. Hui, E. Doerffler, M.O. Clarke, K. Chun, L. Zhang, S. Neville, E. Carra, W. Lew, B. Ross, Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo [2, 1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses, ACS Publications, 2017. [DOI] [PubMed]
- 41.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R.T. Eastman, J.S. Roth, K.R. Brimacombe, A. Simeonov, M. Shen, S. Patnaik, M.D. Hall, Remdesivir: A Review of Its Discovery and Development Leading to Human Clinical Trials for Treatment of COVID-19, (2020). [DOI] [PMC free article] [PubMed]
- 43.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2):e00221–e318. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.C.J. Gordon, E.P. Tchesnokov, E. Woolner, J.K. Perry, J.Y. Feng, D.P. Porter, M. Gotte, Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, Journal of Biological Chemistry (2020) jbc. RA120. 013679. [DOI] [PMC free article] [PubMed]
- 47.Fung A., Jin Z., Dyatkina N., Wang G., Beigelman L., Deval J. Efficiency of incorporation and chain termination determines the inhibition potency of 2'-modified nucleotide analogs against hepatitis C virus polymerase. Antimicrob. Agents Chemother. 2014;58(7):3636–3645. doi: 10.1128/AAC.02666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of Ebola Virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11(4) doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouvet M., Imbert I., Subissi L., Gluais L., Canard B., Decroly E. RNA 3'-end mismatch excision by the severe acute respiratory syndrome coronavirus nonstructural protein nsp10/nsp14 exoribonuclease complex. Proc. Natl. Acad. Sci. 2012;109(24):9372–9377. doi: 10.1073/pnas.1201130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minskaia E., Hertzig T., Gorbalenya A.E., Campanacci V., Cambillau C., Canard B., Ziebuhr J. Discovery of an RNA virus 3′→ 5′ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc. Natl. Acad. Sci. 2006;103(13):5108–5113. doi: 10.1073/pnas.0508200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shannon A., Le N.T.T., Selisko B., Eydoux C., Alvarez K., Guillemot J.-C., Decroly E., Peersen O., Ferron F., Canard B. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020;104793 doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.C.J. Gordon, E.P. Tchesnokov, E. Woolner, J.K. Perry, J.Y. Feng, D.P. Porter, M. Gotte, Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J Journal of Biological Chemistry (2020) jbc. RA120. 013679. [DOI] [PMC free article] [PubMed]
- 53.A. Shannon, N.T.T. Le, B. Selisko, C. Eydoux, K. Alvarez, J.-C. Guillemot, E. Decroly, O. Peersen, F. Ferron, B.J.A.R. Canard, Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites, (2020) 104793. [DOI] [PMC free article] [PubMed]
- 54.C. Liang, L. Tian, Y. Liu, N. Hui, G. Qiao, H. Li, Z. Shi, Y. Tang, D. Zhang, X.J.E.J.o.M.C. Xie, A Promising Antiviral Candidate Drug for the COVID-19 Pandemic: A Mini-Review of Remdesivir, (2020) 112527. [DOI] [PMC free article] [PubMed]
- 55.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. J. New Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. J. Lancet. 2020 doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hillaker E., Belfer J.J., Bondici A., Murad H., Dumkow L.E. Delayed initiation of remdesivir in a COVID-19-positive patient. J. Pharmacother. 2020 doi: 10.1002/phar.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.A.P. Grollman, Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics, Proceedings of the National Academy of Sciences of the United States of America 56(6) (1966) 1867. [DOI] [PMC free article] [PubMed]
- 59.Yang S., Xu M., Lee E.M., Gorshkov K., Shiryaev S.A., He S., Sun W., Cheng Y.-S., Hu X., Tharappel A.M. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov. 2018;4(1):1–14. doi: 10.1038/s41421-018-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan G.T., Kinghorn A., Hughes S., Pezzuto J. Psychotrine and its O-methyl ether are selective inhibitors of human immunodeficiency virus-1 reverse transcriptase. J. Biol. Chem. 1991;266(35):23529–23536. [PubMed] [Google Scholar]
- 61.Mukhopadhyay R., Roy S., Venkatadri R., Su Y.-P., Ye W., Barnaeva E., Griner L.M., Southall N., Hu X., Wang A.Q. Efficacy and mechanism of action of low dose emetine against human cytomegalovirus. PLoS Pathog. 2016;12(6) doi: 10.1371/journal.ppat.1005717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacGibeny M.A., Koyuncu O.O., Wirblich C., Schnell M.J., Enquist L.W. Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons. PLoS Pathog. 2018;14(7) doi: 10.1371/journal.ppat.1007188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Q., Fu Q., Li S., Li J., Liu S., Wang Z., Su Z., Song J., Lu D. Emetine exhibits anticancer activity in breast cancer cells as an antagonist of Wnt/betacatenin signaling. Oncol. Rep. 2019;42(5):1735–1744. doi: 10.3892/or.2019.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choy K.-T., Wong A.-Y.-L., Kaewpreedee P., Sia S.-F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.-P.-H., Huang X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;104786 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patterson J.L., Fernandez-Larsson R. Molecular mechanisms of action of ribavirin. J. Rev. Infect. Dis. 1990;12(6):1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- 66.Borden K.L., Culjkovic-Kraljacic B. Ribavirin as an anti-cancer therapy: acute myeloid leukemia and beyond? Leuk Lymphoma. 2010;51(10):1805–1815. doi: 10.3109/10428194.2010.496506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crotty S., Cameron C., Andino R. Ribavirin's antiviral mechanism of action: lethal mutagenesis? J. Mol. Med. 2002;80(2):86–95. doi: 10.1007/s00109-001-0308-0. [DOI] [PubMed] [Google Scholar]
- 68.Kozhevnikova E.N., van der Knaap J.A., Pindyurin A.V., Ozgur Z., van Ijcken W.F., Moshkin Y.M., Verrijzer C.P. Metabolic enzyme IMPDH is also a transcription factor regulated by cellular state. J. Mol. Cell. Biol. 2012;47(1):133–139. doi: 10.1016/j.molcel.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 69.Thomas E., Ghany M.G., Liang T.J. Chemotherapy, The application and mechanism of action of ribavirin in therapy of hepatitis C. J. Antiviral Chem. 2012;23(1):1–12. doi: 10.3851/IMP2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carrillo-Bustamante P., Nguyen T.H.T., Oestereich L., Günther S., Guedj J., Graw F. Determining Ribavirin’s mechanism of action against Lassa virus infection. J. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-10198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mondelli M.U. The multifaceted functions of ribavirin: antiviral, immunomodulator, or both? J. Hepatol. 2014;60(4):1126–1129. doi: 10.1002/hep.27186. [DOI] [PubMed] [Google Scholar]
- 72.Khalili J.S., Zhu H., Mak A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for evaluation concerning COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Onrust S.V., Lamb H.M. Valrubicin. J. Drugs Aging. 1999;15(1):69–75. doi: 10.2165/00002512-199915010-00006. [DOI] [PubMed] [Google Scholar]
- 74.Hajian R., Hossaini P., Mehrayin Z., Woi P.M., Shams N. DNA-binding studies of valrubicin as a chemotherapy drug using spectroscopy and electrochemical techniques. J. Pharm. Anal. 2017;7(3):176–180. doi: 10.1016/j.jpha.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yaqub F. Mechanism of action of anthracycline drugs. J. Lancet Oncol. 2013;14(8) [Google Scholar]
- 76.Bhattacharya B., Mukherjee S. Cancer therapy using antibiotics. J. Cancer Ther. 2015;6(10):849. [Google Scholar]
- 77.Andersen S.M., Rosada C., Dagnaes-Hansen F., Laugesen I.G., de Darkó E., Dam T.N., Stenderup K. Topical application of valrubicin has a beneficial effect on developing skin tumors. J. Carcinogenesis. 2010;31(8):1483–1490. doi: 10.1093/carcin/bgq122. [DOI] [PubMed] [Google Scholar]
- 78.Rismanbaf A. Potential treatments for COVID-19; a narrative literature review. J. Arch. Acad. Emerg. Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 79.M. Prajapat, P. Sarma, N. Shekhar, P. Avti, S. Sinha, H. Kaur, S. Kumar, A. Bhattacharyya, H. Kumar, S.J.I.j.o.p. Bansal, Drug targets for corona virus: A systematic review, 52(1) (2020) 56. [DOI] [PMC free article] [PubMed]
- 80.Gutierrez A., Pan L., Groen R.W., Baleydier F., Kentsis A., Marineau J., Grebliunaite R., Kozakewich E., Reed C., Pflumio F. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J. Clin. Investig. 2014;124(2):644–655. doi: 10.1172/JCI65093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X., Wang X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genomics. 2020 doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paudler W.W., Kerley G.I., McKay J. The alkaloids of Cephalotaxus drupacea and Cephalotaxus fortunei. J. Organ. Chem. 1963;28(9):2194–2197. [Google Scholar]
- 83.Lü S., Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J. Hematol. Oncol. 2014;7(1):2. doi: 10.1186/1756-8722-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Army C.P.s.L. 187th Hospital: homoharringtonine in the treatment of leukemias: clinical analysis of 72 cases. Chinese Med. J. 1978;3:163–166. [Google Scholar]
- 85.S. O'Brien, H. Kantarjian, M. Keating, M. Beran, C. Koller, L. Robertson, J. Hester, M. Rios, M. Andreeff, M. Talpaz, Homoharringtonine therapy induces responses in patients with chronic myelogenous leukemia in late chronic phase, (1995). [PubMed]
- 86..S. Lam, E.S. Ho, B.-L. He, W.-W. Wong, C.-Y. Cher, N.K. Ng, C.-H. Man, H. Gill, A.M. Cheung, H.-W. Ip, Homoharringtonine (omacetaxine mepesuccinate) as an adjunct for FLT3-ITD acute myeloid leukemia, Science translational medicine 8(359) (2016) 359ra129-359ra129. [DOI] [PubMed]
- 87.Fresno M., Jiménez A., Vázquez D. Inhibition of translation in eukaryotic systems by harringtonine. Eur. J. Biochem. 1977;72(2):323–330. doi: 10.1111/j.1432-1033.1977.tb11256.x. [DOI] [PubMed] [Google Scholar]
- 88.Killian B.J., Kravitz J.Y., Somani S., Dasgupta P., Pang Y.-P., Gilson M.K. Configurational entropy in protein-peptide binding: computational study of Tsg101 ubiquitin E2 variant domain with an HIV-derived PTAP nonapeptide. J. Mol. Biol. 2009;389(2):315–335. doi: 10.1016/j.jmb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.K.T. Choy, A.Y. Wong, P. Kaewpreedee, S.F. Sia, D. Chen, K.P.Y. Hui, D.K.W. Chu, M.C.W. Chan, P.P. Cheung, X. Huang, M. Peiris, H.L. Yen, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral Res 178 104786. [DOI] [PMC free article] [PubMed]
- 90.White C., Alshaker H., Cooper C., Winkler M., Pchejetski D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget. 2016;7(17):23106–23127. doi: 10.18632/oncotarget.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y., Li X., Ciric B., Ma C.-G., Gran B., Rostami A., Zhang G.-X. Effect of fingolimod on neural stem cells: a novel mechanism and broadened application for neural repair. J. Mol. Ther. 2017;25(2):401–415. doi: 10.1016/j.ymthe.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehling M., Kappos L., Derfuss T. Fingolimod for multiple sclerosis: mechanism of action, clinical outcomes, and future directions. J. Curr. Neurol. Neurosci. Rep. 2011;11(5):492. doi: 10.1007/s11910-011-0216-9. [DOI] [PubMed] [Google Scholar]
- 93.Ricklin M.E., Lorscheider J., Waschbisch A., Paroz C., Mehta S.K., Pierson D.L., Kuhle J., Fischer-Barnicol B., Sprenger T., Lindberg R.L. T-cell response against varicella-zoster virus in fingolimod-treated MS patients. J. Neurol. 2013;81(2):174–181. doi: 10.1212/WNL.0b013e31829a3311. [DOI] [PubMed] [Google Scholar]
- 94.A.T. Reder, COVID-19 and multiple sclerosis.
- 95.Li W., Xu H., Testai F.D. Mechanism of action and clinical potential of fingolimod for the treatment of stroke. J. Front. Neurol. 2016;7:139. doi: 10.3389/fneur.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hartung E.F. History of the use of colchicum and related medicaments in gout: with suggestions for further research. J. Ann. Rheumatic Dis. 1954;13(3):190. doi: 10.1136/ard.13.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dalbeth N., Lauterio T.J., Wolfe H.R. Mechanism of action of colchicine in the treatment of gout. J. Clin. Ther. 2014;36(10):1465–1479. doi: 10.1016/j.clinthera.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 98.Zhang T., Chen W., Jiang X., Liu L., Wei K., Du H., Wang H., Li J. Anticancer effects and underlying mechanism of Colchicine on human gastric cancer cell lines in vitro and in vivo. Biosci. Rep. 2019;39(1) doi: 10.1042/BSR20181802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swanson K.V., Deng M., Ting J.P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. J. Nature Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robertson S., Martínez G.J., Payet C.A., Barraclough J.Y., Celermajer D.S., Bursill C., Patel S. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. J. Clin. Sci. 2016;130(14):1237–1246. doi: 10.1042/CS20160090. [DOI] [PubMed] [Google Scholar]
- 101.Moossavi M., Parsamanesh N., Bahrami A., Atkin S.L., Sahebkar A. Role of the NLRP3 inflammasome in cancer. J. Mol. Cancer. 2018;17(1):158. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vitiello A., Ferrara F., Pelliccia C., Granata G., La Porta R. Cytokine storm and colchicine potential role fighting SARS-CoV-2 pneumonia. J. Italian J. Med. 2020 [Google Scholar]
- 103.Deftereos S.G., Siasos G., Giannopoulos G., Vrachatis D.A., Angelidis C., Giotaki S.G., Gargalianos P., Giamarellou H., Gogos C., Daikos G., Lazanas M., Lagiou P., Saroglou G., Sipsas N., Tsiodras S., Chatzigeorgiou D., Moussas N., Kotanidou A., Koulouris N., Oikonomou E., Kaoukis A., Kossyvakis C., Raisakis K., Fountoulaki K., Comis M., Tsiachris D., Sarri E., Theodorakis A., Martinez-Dolz L., Sanz-Sanchez J., Reimers B., Stefanini G.G., Cleman M., Filippou D., Olympios C.D., Pyrgakis V.N., Goudevenos J., Hahalis G., Kolettis T.M., Iliodromitis E., Tousoulis D., Stefanadis C. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J. Cardiol. 2020 doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heo T.-H., Wahler J., Suh N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget. 2016;7(13):15460. doi: 10.18632/oncotarget.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oguro T., Ishibashi K., Sugino T., Hashimoto K., Tomita S., Takahashi N., Yanagida T., Haga N., Aikawa K., Suzutani T. Humanised antihuman IL-6R antibody with interferon inhibits renal cell carcinoma cell growth in vitro and in vivo through suppressed SOCS3 expression. Eur. J. Cancer. 2013;49(7):1715–1724. doi: 10.1016/j.ejca.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 106.Korkaya H., Kim G.-I., Davis A., Malik F., Henry N.L., Ithimakin S., Quraishi A.A., Tawakkol N., D'Angelo R., Paulson A.K. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell. 2012;47(4):570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 111.Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 112.Sheppard M., Laskou F., Stapleton P.P., Hadavi S., Dasgupta B. Taylor & Francis; 2017. Tocilizumab (Actemra) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao X., Huang J., Zhong H., Shen N., Faggioni R., Fung M., Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014;141(2):125–139. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 114.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huizinga T.W., Fleischmann R.M., Jasson M., Radin A.R., van Adelsberg J., Fiore S., Huang X., Yancopoulos G.D., Stahl N., Genovese M.C. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann. Rheum. Dis. 2014;73(9):1626–1634. doi: 10.1136/annrheumdis-2013-204405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang L., Luan B., Adler A., Eichten A., Daly C., Thurston G. Sarilumab (REGN88), a fully-human anti-IL6R antibody, inhibits tumor growth in preclinical models, as a single agent and in combination with the VEGF blocker aflibercept. AACR. 2012 [Google Scholar]
- 117.Langhoff E., Ladefoged J. Relative immunosuppressive potency of various corticosteroids measured in vitro. Eur. J. Clin. Pharmacol. 1983;25(4):459–462. doi: 10.1007/BF00542111. [DOI] [PubMed] [Google Scholar]
- 118.Leneva I.A., Russell R.J., Boriskin Y.S., Hay A.J. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 119.Pecheur E.-I., Lavillette D., Alcaras F., Molle J., Boriskin Y.S., Roberts M., Cosset F.-L., Polyak S.J. Biochemical mechanism of hepatitis C virus inhibition by the broad-spectrum antiviral arbidol. Biochemistry. 2007;46(20):6050–6059. doi: 10.1021/bi700181j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhong Z., Zhang Q., Xia H., Wang A., Liang W., Zhou W., Zhou L., Liu X., Rao L., Li Z. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am. J. Transplant. 2020 doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu L., Gong N., Liu B., Lu X., Chen D., Chen S., Shu H., Ma K., Xu X., Guo Z. Coronavirus Disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur. Urol. 2020 doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cruden N.L., Newby D.E. Therapeutic potential of icatibant (HOE-140, JE-049) Expert Opin. Pharmacother. 2008;9(13):2383–2390. doi: 10.1517/14656566.9.13.2383. [DOI] [PubMed] [Google Scholar]
- 124.van de Veerdonk F.L., Netea M.G., van Deuren M., van der Meer J.W.M., de Mast Q., Brüggemann R.J., van der Hoeven H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife. 2020;9 doi: 10.7554/eLife.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hock F., Wirth K., Albus U., Linz W., Gerhards H., Wiemer G., Henke S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br. J. Pharmacol. 1991;102(3):769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wirth K., Hock F., Albus U., Linz W., Alpermann H., Anagnostopoulos H., Henke S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br. J. Pharmacol. 1991;102(3):774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.F. van de Veerdonk, M.G. Netea, M. van Deuren, J.W. van der Meer, Q. de Mast, R.J. Bruggemann, H. van der Hoeven, Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach, (2020).
- 128.J.A. Roche, R. Roche, A hypothesized role for dysregulated bradykinin signaling in COVID‐19 respiratory complications, The FASEB Journal. [DOI] [PMC free article] [PubMed]
- 129.Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S., McCray P.B., Jr, Chappell M., Hackam D.J., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018;314(1):L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bardsley-Elliot A., Noble S. Oseltamivir. Drugs. 1999;58(5):851–860. doi: 10.2165/00003495-199958050-00007. [DOI] [PubMed] [Google Scholar]
- 131.Treanor J.J., Hayden F.G., Vrooman P.S., Barbarash R., Bettis R., Riff D., Singh S., Kinnersley N., Ward P., Mills R.G. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283(8):1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 132.McKimm-Breschkin J.L. Management of influenza virus infections with neuraminidase inhibitors. Treatments Respiratory Med. 2005;4(2):107–116. doi: 10.2165/00151829-200504020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moscona A. Oseltamivir resistance—disabling our influenza defenses. N. Engl. J. Med. 2005;353(25):2633–2636. doi: 10.1056/NEJMp058291. [DOI] [PubMed] [Google Scholar]
- 134.de Oliveira J.T., Santos A.L., Gomes C., Barros R., Ribeiro C., Mendes N., de Matos A.J., Vasconcelos M.H., Oliveira M.J., Reis C.A., Gartner F. Anti-influenza neuraminidase inhibitor oseltamivir phosphate induces canine mammary cancer cell aggressiveness. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Revista Panamericana de Salud Pública. 2020;44 doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Inan A., Sen M., Sürgit Ö., Ergin M., Bozer M. Effects of the histamine H2 receptor antagonist famotidine on the healing of colonic anastomosis in rats. Clinics. 2009;64(6):567–570. doi: 10.1590/S1807-59322009000600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miyata K., Kamato T., Nishida A., Honda K. Studies on the mechanism for the gastric mucosal protection by famotidine in rats. Japanese J. Pharmacol. 1991;55(2):211–222. doi: 10.1254/jjp.55.211. [DOI] [PubMed] [Google Scholar]
- 139.Janowitz T., Gablenz E., Pattinson D., Wang T.C., Conigliaro J., Tracey K., Tuveson D. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020 doi: 10.1136/gutjnl-2020-321852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.D.E. Freedberg, J. Conigliaro, T.C. Wang, K.J. Tracey, M.V. Callahan, J.A. Abrams, G. Famotidine Research Group Famotidine Research, M.E. Sobieszczyk, D.D. Markowitz, A. Gupta, M.R. O'Donnell, J. Li, D.A. Tuveson, Z. Jin, W.C. Turner, D.W. Landry, Famotidine Use is Associated with Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study, Gastroenterology (2020). [DOI] [PMC free article] [PubMed]
- 141.Lopez-Lopez J., Perez-Garcia M., Canet E., Gonzalez C. Effects of almitrine bismesylate on the ionic currents of chemoreceptor cells from the carotid body. Mol. Pharmacol. 1998;53(2):330–339. doi: 10.1124/mol.53.2.330. [DOI] [PubMed] [Google Scholar]
- 142.Esposito V., Verdina A., Manente L., Spugnini E.P., Viglietti R., Parrella R., Pagliano P., Parrella G., Galati R., De Luca A. Amprenavir inhibits the migration in human hepatocarcinoma cell and the growth of xenografts. J. Cell. Physiol. 2013;228(3):640–645. doi: 10.1002/jcp.24173. [DOI] [PubMed] [Google Scholar]
- 143.Helsley R.N., Sui Y., Ai N., Park S.-H., Welsh W.J., Zhou C. Pregnane X receptor mediates dyslipidemia induced by the HIV protease inhibitor amprenavir in mice. Mol. Pharmacol. 2013;83(6):1190–1199. doi: 10.1124/mol.113.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lv Z., Chu Y., Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV/AIDS (Auckland, NZ) 2015;7:95. doi: 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gan X., Liu D., Ge M., Luo C., Gao W., Hei Z. Treatment of mice with cromolyn sodium after reperfusion, but not prior to ischemia, attenuates small intestinal ischemia-reperfusion injury. Mol. Med. Rep. 2013;8(3):928–934. doi: 10.3892/mmr.2013.1591. [DOI] [PubMed] [Google Scholar]
- 146.Murphy S., Kelly H.W. Cromolyn sodium: a review of mechanisms and clinical use in asthma. Drug Intelligence Clin. Pharmacy. 1987;21(1):22–35. doi: 10.1177/10600280870211p102. [DOI] [PubMed] [Google Scholar]
- 147.Motawi T.M., Bustanji Y., EL-Maraghy S.A., Taha M.O., Al Ghussein M.A. Naproxen and cromolyn as new glycogen synthase kinase 3β inhibitors for amelioration of diabetes and obesity: an investigation by docking simulation and subsequent in vitro/in vivo biochemical evaluation. J. Biochem. Mol. Toxicol. 2013;27(9):425–436. doi: 10.1002/jbt.21503. [DOI] [PubMed] [Google Scholar]
- 148.Arumugam T., Ramachandran V., Logsdon C.D. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J. Natl. Cancer Inst. 2006;98(24):1806–1818. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Granucci E.J., Griciuc A., Mueller K.A., Mills A.N., Le H., Dios A.M., McGinty D., Pereira J., Elmaleh D., Berry J.D., Paganoni S., Cudkowicz M.E., Tanzi R.E., Sadri-Vakili G. Cromolyn sodium delays disease onset and is neuroprotective in the SOD1(G93A) Mouse Model of amyotrophic lateral sclerosis. Sci. Rep. 2019;9(1):17728. doi: 10.1038/s41598-019-53982-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aggarwal V., Tuli H.S., Thakral F., Singhal P., Aggarwal D., Srivastava S., Pandey A., Sak K., Varol M., Khan M.A. Molecular mechanisms of action of hesperidin in cancer: recent trends and advancements. Exp. Biol. Med. 2020;245(5):486–497. doi: 10.1177/1535370220903671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pinho-Ribeiro F.A., Hohmann M.S., Borghi S.M., Zarpelon A.C., Guazelli C.F., Manchope M.F., Casagrande R., Verri W.A., Jr Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: role of TRPV1, oxidative stress, cytokines and NF-κB. Chem. Biol. Interact. 2015;228:88–99. doi: 10.1016/j.cbi.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 152.Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS-CoV-2 by blocking the trimerization of viral spike glycoprotein? Int. J. Antimicrob. Agents. 2020;105998 doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Musarrat F., Chouljenko V., Dahal A., Nabi R., Chouljenko T., Jois S.D., Kousoulas K.G. The anti-HIV Drug Nelfinavir Mesylate (Viracept) is a Potent Inhibitor of Cell Fusion Caused by the SARS-CoV-2 Spike (S) Glycoprotein Warranting further Evaluation as an Antiviral against COVID-19 infections. J. Med. Virol. 2020 doi: 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;105938 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoffmann M., Mösbauer K., Hofmann-Winkler H., Kaul A., Kleine-Weber H., Krüger N., Gassen N.C., Müller M.A., Drosten C., Pöhlmann S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 158.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2(1):1–10. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Asghari A., Naseri M., Safari H., Saboory E., Parsamanesh N. The novel insight of SARS-CoV-2 molecular biology and pathogenesis and therapeutic options. DNA Cell Biol. 2020 doi: 10.1089/dna.2020.5703. [DOI] [PubMed] [Google Scholar]
- 160.Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9(5):1267. doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020;104859 doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.V. Monteil, H. Kwon, P. Prado, A. Hagelkruys, R.A. Wimmer, M. Stahl, A. Leopoldi, E. Garreta, C. Hurtado Del Pozo, F. Prosper, J.P. Romero, G. Wirnsberger, H. Zhang, A.S. Slutsky, R. Conder, N. Montserrat, A. Mirazimi, J.M. Penninger, Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2, Cell 181(4) (2020) 905-913 e7. [DOI] [PMC free article] [PubMed]
- 163.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U.S.A. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ahmed S., Zhou Z., Zhou J., Chen S.Q. Pharmacogenomics of drug metabolizing enzymes and transporters: relevance to precision medicine. Genomics Proteomics Bioinf. 2016;14(5):298–313. doi: 10.1016/j.gpb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kay K., Hastings I.M. Improving pharmacokinetic-pharmacodynamic modeling to investigate anti-infective chemotherapy with application to the current generation of antimalarial drugs. PLoS Comput. Biol. 2013;9(7) doi: 10.1371/journal.pcbi.1003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;104787 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S. Remdesivir for the treatment of Covid-19—preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 170.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.M.G.S. Borba, F.F.A. Val, V.S. Sampaio, M.A.A. Alexandre, G.C. Melo, M. Brito, M.P.G. Mourão, J.D. Brito-Sousa, D. Baía-da-Silva, M.V.F. Guerra, Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial, JAMA network open 3(4) (2020) e208857-e208857. [DOI] [PubMed]
- 172.Watson J.A., Tarning J., Hoglund R.M., Baud F.J., Megarbane B., Clemessy J.-L., White N.J. Concentration-dependent mortality of chloroquine in overdose. Elife. 2020;9 doi: 10.7554/eLife.58631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Piszczatoski C.R., Powell J. Emergency approval of chloroquine and hydroxychloroquine for treatment of COVID-19. Ann. Pharmacother. 2020;1060028020925558 doi: 10.1177/1060028020925558. [DOI] [PubMed] [Google Scholar]
- 174.Di Castelnuovo A., Costanzo S., Antinori A., Berselli N., Blandi L., Bruno R., Cauda R., Guaraldi G., Menicanti L., My I. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur. J. Internal Med. 2020 doi: 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lane J.C., Weaver J., Kostka K., Duarte-Salles T., Abrahao M.T.F., Alghoul H., Alser O., Alshammari T.M., Biedermann P., Banda J.M. Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu J., Zheng X., Huang Y., Shan H., Huang J. Successful use of methylprednisolone for treating severe COVID-19. J. Allergy Clin. Immunol. 2020;146(2):325–327. doi: 10.1016/j.jaci.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.F. Salton, P. Confalonieri, P. Santus, S. Harari, R. Scala, S. Lanini, V. Vertui, T. Oggionni, A. Caminati, V. Patruno, Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia, medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 178.Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P., Dong N., Tong Q. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Trans. Targeted Ther. 2020;5(1):1–3. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.S.G. Deftereos, G. Giannopoulos, D.A. Vrachatis, G.D. Siasos, S.G. Giotaki, P. Gargalianos, S. Metallidis, G. Sianos, S. Baltagiannis, P. Panagopoulos, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA network open 3(6) (2020) e2013136-e2013136. [DOI] [PMC free article] [PubMed]
- 180.M.I.F. Lopes, L.P. Bonjorno, M.C. Giannini, N.B. Amaral, M.N. Benatti, U.C. Rezek, L.L. Emrich-Filho, B.A. Sousa, S.C. Almeida, R. Luppino-Assad, Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial, medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 181.Freedberg D.E., Conigliaro J., Wang T.C., Tracey K.J., Callahan M.V., Abrams J.A., Sobieszczyk M.E., Markowitz D.D., Gupta A., O’Donnell M.R. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.M. Loffredo, H. Lucero, D.-Y. Chen, A. O'Connell, S. Bergqvist, A. Munawar, A. Bandara, S. DeGraef, S.D. Weeks, F. Douam, The Effect of famotidine on SARS-CoV-2 proteases and virus replication, bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 183.Yamamoto N., Matsuyama S., Hoshino T., Yamamoto N. Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. BioRxiv. 2020 [Google Scholar]
- 184.Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B., Zaslavsky L., Zhang J., Bolton E.E. PubChem 2019 update: improved access to chemical data. Nucl. Acids Res. 2019;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Checker R., Sandur S.K., Sharma D., Patwardhan R.S., Jayakumar S., Kohli V., Sethi G., Aggarwal B.B., Sainis K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Habtemariam S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxidative Med. Cell. Longevity. 2019;2019 doi: 10.1155/2019/8512048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim S.O., Park J.Y., Jeon S.Y., Yang C.H., Kim M.R. Saikosaponin a, an active compound of Radix Bupleuri, attenuates inflammation in hypertrophied 3T3-L1 adipocytes via ERK/NF-κB signaling pathways. Int. J. Mol. Med. 2015;35(4):1126–1132. doi: 10.3892/ijmm.2015.2093. [DOI] [PubMed] [Google Scholar]
- 188.Gao H., Song Y., Li D., Feng W., Liu J. Saikosaponin A inhibits IL-1β-induced inflammatory mediators in human osteoarthritis chondrocytes by activating LXRα. Oncotarget. 2017;8(51):88941. doi: 10.18632/oncotarget.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu C.-N., Yuan Z.-G., Zhang X.-L., Yan R., Zhao Y.-Q., Liao M., Chen J.-X. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-κB signaling pathway. Int. Immunopharmacol. 2012;14(1):121–126. doi: 10.1016/j.intimp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 190.Wang C.P., Zhang L.Z., Li G.C., Shi Y.W., Li J.L., Zhang X.C., Wang Z.W., Ding F., Liang X.M. Mulberroside a protects against ischemic impairment in primary culture of rat cortical neurons after oxygen–glucose deprivation followed by reperfusion. J. Neurosci. Res. 2014;92(7):944–954. doi: 10.1002/jnr.23374. [DOI] [PubMed] [Google Scholar]
- 191.Lim K.M., An S., Lee O.K., Lee M.J., Lee J.P., Lee K.S., Lee G.T., Lee K.K., Bae S. Analysis of changes in microRNA expression profiles in response to the troxerutin-mediated antioxidant effect in human dermal papilla cells. Mol. Med. Rep. 2015;12(2):2650–2660. doi: 10.3892/mmr.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang Z., Wang X., Zheng G., Shan Q., Lu J., Fan S., Sun C., Wu D., Zhang C., Su W. Troxerutin attenuates enhancement of hepatic gluconeogenesis by inhibiting NOD activation-mediated inflammation in high-fat diet-treated mice. Int. J. Mol. Sci. 2017;18(1):31. doi: 10.3390/ijms18010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Badalzadeh R., Layeghzadeh N., Alihemmati A., Mohammadi M. Beneficial effect of troxerutin on diabetes-induced vascular damages in rat aorta: histopathological alterations and antioxidation mechanism. Int. J. Endocrinol. Metab. 2015;13(2) doi: 10.5812/ijem.25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Herbert J., Maffrand J., Taoubi K., Augereau J., Fouraste I., Gleye J. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J. Nat. Prod. 1991;54(6):1595–1600. doi: 10.1021/np50078a016. [DOI] [PubMed] [Google Scholar]
- 195.Yoo K.H., Park J.H., Lee D.Y., Hwang-Bo J., Baek N.I., Chung I.S. Corosolic acid exhibits anti-angiogenic and anti-lymphangiogenic effects on in vitro endothelial cells and on an in vivo CT-26 colon carcinoma animal model. Phytother. Res. 2015;29(5):714–723. doi: 10.1002/ptr.5306. [DOI] [PubMed] [Google Scholar]
- 196.Ahn K.-S., Hahm M.S., Park E.J., Lee H.-K., Kim I.-H. Corosolic acid isolated from the fruit of Crataegus pinnatifida var. psilosa is a protein kinase C inhibitor as well as a cytotoxic agent. Planta Med. 1998;64(05):468–470. doi: 10.1055/s-2006-957487. [DOI] [PubMed] [Google Scholar]
- 197.Zou Y., Zhang M., Zhang T., Wu J., Wang J., Liu K., Zhan N. Antioxidant and anti-inflammatory activities of cynaroside from Elsholtiza bodinieri. Nat. Prod. Commun. 2018;13(11) 1934578X1801301122. [Google Scholar]
- 198.Sun X., Sun G.B., Wang M., Xiao J., Sun X.B. Protective effects of cynaroside against H2O2-induced apoptosis in H9c2 cardiomyoblasts. J. Cell. Biochem. 2011;112(8):2019–2029. doi: 10.1002/jcb.23121. [DOI] [PubMed] [Google Scholar]
- 199.Dhakal H., Lee S., Choi J.K., Kwon T.K., Khang D., Kim S.-H. Inhibitory effects of orientin in mast cell-mediated allergic inflammation. Pharmacol. Rep. 2020:1–9. doi: 10.1007/s43440-019-00048-3. [DOI] [PubMed] [Google Scholar]
- 200.Piver B., Berthou F., Dreano Y., Lucas D. Differential inhibition of human cytochrome P450 enzymes by ε-viniferin, the dimer of resveratrol: comparison with resveratrol and polyphenols from alcoholized beverages. Life Sci. 2003;73(9):1199–1213. doi: 10.1016/s0024-3205(03)00420-x. [DOI] [PubMed] [Google Scholar]
- 201.Vitrac X., Bornet A., Vanderlinde R., Valls J., Richard T., Delaunay J.-C., Mérillon J.-M., Teissédre P.-L. Determination of stilbenes (δ-viniferin, trans-astringin, trans-piceid, cis-and trans-resveratrol, ε-viniferin) in Brazilian wines. J. Agric. Food. Chem. 2005;53(14):5664–5669. doi: 10.1021/jf050122g. [DOI] [PubMed] [Google Scholar]
- 202.Hwang I.-W., Chung S.-K. Isolation and identification of myricitrin, an antioxidant flavonoid, from daebong persimmon peel. Preven. Nutr. Food Sci. 2018;23(4):341. doi: 10.3746/pnf.2018.23.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease$ J. Biomol. Struct. Dyn. (just-accepted) 2020:1–16. doi: 10.1080/07391102.2020.1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lin M., Li L., Li L., Pokhrel G., Qi G., Rong R., Zhu T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complementary Alternative Med. 2014;14(1):19. doi: 10.1186/1472-6882-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim D.K. Inhibitory effect of corynoline isolated from the aerial parts ofcorydalis incisa on the acetylcholinesterase. Arch. Pharmacal Res. 2002;25(6):817. doi: 10.1007/BF02976997. [DOI] [PubMed] [Google Scholar]
- 206.Liu B., Su K., Wang J., Wang J., Xin Z., Li F., Fu Y. Corynoline exhibits anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cells through activating Nrf 2. Inflammation. 2018;41(5):1640–1647. doi: 10.1007/s10753-018-0807-6. [DOI] [PubMed] [Google Scholar]
- 207.Wu Y., Nie Y., Huang J., Qiu Y., Wan B., Liu G., Chen J., Chen D., Pang Q. Protostemonine alleviates heat-killed methicillin-resistant Staphylococcus aureus-induced acute lung injury through MAPK and NF-κB signaling pathways. Int. Immunopharmacol. 2019;77 doi: 10.1016/j.intimp.2019.105964. [DOI] [PubMed] [Google Scholar]
- 208.Wu Y.-X., He H.-Q., Nie Y.-J., Ding Y.-H., Sun L., Qian F. Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacol. Sin. 2018;39(1):85–96. doi: 10.1038/aps.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liczbiński P., Bukowska B. Molecular mechanism of amygdalin action in vitro: review of the latest research. Immunopharmacol. Immunotoxicol. 2018;40(3):212–218. doi: 10.1080/08923973.2018.1441301. [DOI] [PubMed] [Google Scholar]
- 210.Tu J., Guo Y., Hong W., Fang Y., Han D., Zhang P., Wang X., Körner H., Wei W. The regulatory effects of paeoniflorin and its derivative paeoniflorin-6′-O-benzene sulfonate CP-25 on inflammation and immune diseases. Front. Pharmacol. 2019;10:57. doi: 10.3389/fphar.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kuo Y.-H., Hwang S.-Y., Kuo L.-M.Y., Lee Y.-L., Li S.-Y., Shen Y.-C. A novel cytotoxic C-methylated biflavone, taiwanhomoflavone-B from the twigs of Cephalotaxus wilsoniana. Chem. Pharm. Bull. 2002;50(12):1607–1608. doi: 10.1248/cpb.50.1607. [DOI] [PubMed] [Google Scholar]
- 212.Bischoff T.A., Kelley C.J., Karchesy Y., Laurantos M., Nguyen-Dinh P., Arefi A.G. Antimalarial activity of Lactucin and Lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 2004;95(2–3):455–457. doi: 10.1016/j.jep.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 213.Selvakumari E., Shantha A., Kumar C.S., Prabhu T.P. Phytochemistry and pharmacology of the genus nymphaea. J. Acad. Ind. Res. (JAIR) 2016;5(7):98. [Google Scholar]
- 214.Roy U., Bulot C., Zu Bentrup K.H., Mondal D. Specific increase in MDR1 mediated drug-efflux in human brain endothelial cells following co-exposure to HIV-1 and saquinavir. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Barillari G., Iovane A., Bacigalupo I., Palladino C., Bellino S., Leone P., Monini P., Ensoli B. Ritonavir or saquinavir impairs the invasion of cervical intraepithelial neoplasia cells via a reduction of MMP expression and activity. Aids. 2012;26(8):909–919. doi: 10.1097/QAD.0b013e328351f7a5. [DOI] [PubMed] [Google Scholar]
- 216.Merry C., Barry M.G., Mulcahy F., Ryan M., Heavey J., Tjia J.F., Gibbons S.E., Breckenridge A.M., Back D.J. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. Aids. 1997;11(4):F29–F33. doi: 10.1097/00002030-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 217.Wang Y.-F., Wang X.-Y., Ren Z., Qian C.-W., Li Y.-C., Kaio K., Wang Q.-D., Zhang Y., Zheng L.-Y., Jiang J.-H. Phyllaemblicin B inhibits Coxsackie virus B3 induced apoptosis and myocarditis. Antiviral Res. 2009;84(2):150–158. doi: 10.1016/j.antiviral.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 218.Sun D.-K., Wang L., Zhang P. Antitumor effects of chrysanthemin in PC-3 human prostate cancer cells are mediated via apoptosis induction, caspase signalling pathway and loss of mitochondrial membrane potential. Afr. J. Tradit. Complement. Altern. Med. 2017;14(4):54–61. doi: 10.21010/ajtcam.v14i4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kikuchi H., Tsukitani Y., Shimizu I., Kobayashi M., Kitagawa I. Marine natural products. XI. An antiinflammatory scalarane-type bishomosesterterpene, foliaspongin, from the Okinawan marine sponge Phyllospongia foliascens (Pallas) Chem. Pharm. Bull. 1983;31(2):552–556. [Google Scholar]
- 220.Ren S., Zhang H., Mu Y., Sun M., Liu P. Pharmacological effects of Astragaloside IV: a literature review. J. Tradit. Chin. Med. 2013;33(3):413–416. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- 221.Yang H., Wang J., Fan J.-H., Zhang Y.-Q., Zhao J.-X., Dai X.-J., Liu Q., Shen Y.-J., Liu C., Sun W.-D. Ilexgenin A exerts anti-inflammation and anti-angiogenesis effects through inhibition of STAT3 and PI3K pathways and exhibits synergistic effects with Sorafenib on hepatoma growth. Toxicol. Appl. Pharmacol. 2017;315:90–101. doi: 10.1016/j.taap.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 222.Aksu E., Kandemir F., Özkaraca M., Ömür A., Küçükler S., Çomaklı S. Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia. 2017;49(1) doi: 10.1111/and.12593. [DOI] [PubMed] [Google Scholar]
- 223.Han Y.-H., Kee J.-Y., Park J., Kim D.-S., Shin S., Youn D.-H., Kang J., Jung Y., Lee Y.-M., Park J.-H. Lipin1-mediated repression of adipogenesis by rutin. Am. J. Chinese Med. 2016;44(03):565–578. doi: 10.1142/S0192415X16500312. [DOI] [PubMed] [Google Scholar]
- 224.Lu N., Ding Y., Yang Z., Gao P. Effects of rutin on the redox reactions of hemoglobin. Int. J. Biol. Macromol. 2016;89:175–180. doi: 10.1016/j.ijbiomac.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 225.Su X., Wu L., Hu M., Dong W., Xu M., Zhang P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomed. Pharmacother. 2017;95:670–678. doi: 10.1016/j.biopha.2017.08.123. [DOI] [PubMed] [Google Scholar]
- 226.Li C., Peng S., Liu X., Han C., Wang X., Jin T., Liu S., Wang W., Xie X., He X. Glycyrrhizin, a direct HMGB1 antagonist, ameliorates inflammatory infiltration in a model of autoimmune thyroiditis via inhibition of TLR2-HMGB1 signaling. Thyroid. 2017;27(5):722–731. doi: 10.1089/thy.2016.0432. [DOI] [PubMed] [Google Scholar]
- 227.Wang L.-Y., Cheng K.C., Li Y., Niu C.-S., Cheng J.-T., Niu H.-S. Glycyrrhizic acid increases glucagon like peptide-1 secretion via TGR5 activation in type 1-like diabetic rats. Biomed. Pharmacother. 2017;95:599–604. doi: 10.1016/j.biopha.2017.08.087. [DOI] [PubMed] [Google Scholar]
- 228.Liu H., Zheng Y.-F., Li C.-Y., Zheng Y.-Y., Wang D.-Q., Wu Z., Huang L., Wang Y.-G., Li P.-B., Peng W. Discovery of anti-inflammatory ingredients in chinese herbal formula kouyanqing granule based on relevance analysis between chemical characters and biological effects. Sci. Rep. 2015;5(1):1–16. doi: 10.1038/srep18080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hu W., Yang X., Zhe C., Zhang Q., Sun L., Cao K. Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-κB activation in LPS-induced RAW264. 7 macrophage cells. Pharmacol. Rep. 2011;63(3):781–789. doi: 10.1016/s1734-1140(11)70590-4. [DOI] [PubMed] [Google Scholar]
- 230.Li H., Wang Q., Dong L., Liu C., Sun Z., Gao L., Wang X. Morusin suppresses breast cancer cell growth in vitro and in vivo through C/EBPβ and PPARγ mediated lipoapoptosis. J. Exp. Clin. Cancer Res. 2015;34(1):137. doi: 10.1186/s13046-015-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lee J.-C., Won S.-J., Chao C.-L., Wu F.-L., Liu H.-S., Ling P., Lin C.-N., Su C.-L. Morusin induces apoptosis and suppresses NF-κB activity in human colorectal cancer HT-29 cells. Biochem. Biophys. Res. Commun. 2008;372(1):236–242. doi: 10.1016/j.bbrc.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 232.Lim S.-L., Park S.-Y., Kang S., Park D., Kim S.-H., Um J.-Y., Jang H.-J., Lee J.-H., Jeong C.-H., Jang J.-H. Morusin induces cell death through inactivating STAT3 signaling in prostate cancer cells. Am. J. Cancer Res. 2015;5(1):289. [PMC free article] [PubMed] [Google Scholar]
- 233.He J., Yu S., Guo C., Tan L., Song X., Wang M., Wu J., Long Y., Gong D., Zhang R. Polyphyllin I induces autophagy and cell cycle arrest via inhibiting PDK1/Akt/mTOR signal and downregulating cyclin B1 in human gastric carcinoma HGC-27 cells. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109189. [DOI] [PubMed] [Google Scholar]
- 234.Liu J., Zhang Y., Chen L., Yu F., Li X., Tao D., Zhao J., Zhou S. Polyphyllin I induces G2/M phase arrest and apoptosis in U251 human glioma cells via mitochondrial dysfunction and the JNK signaling pathway. Acta Biochim. Biophys. Sin. 2017;49(6):479–486. doi: 10.1093/abbs/gmx033. [DOI] [PubMed] [Google Scholar]
- 235.Kong M., Fan J., Dong A., Cheng H., Xu R. Effects of polyphyllin I on growth inhibition of human non-small lung cancer cells and in xenograft. Acta Biochim. Biophys. Sin. 2010;42(11):827–833. doi: 10.1093/abbs/gmq091. [DOI] [PubMed] [Google Scholar]