Fig. 3. Rapid protein labeling with various amine-derivatized functionalities.
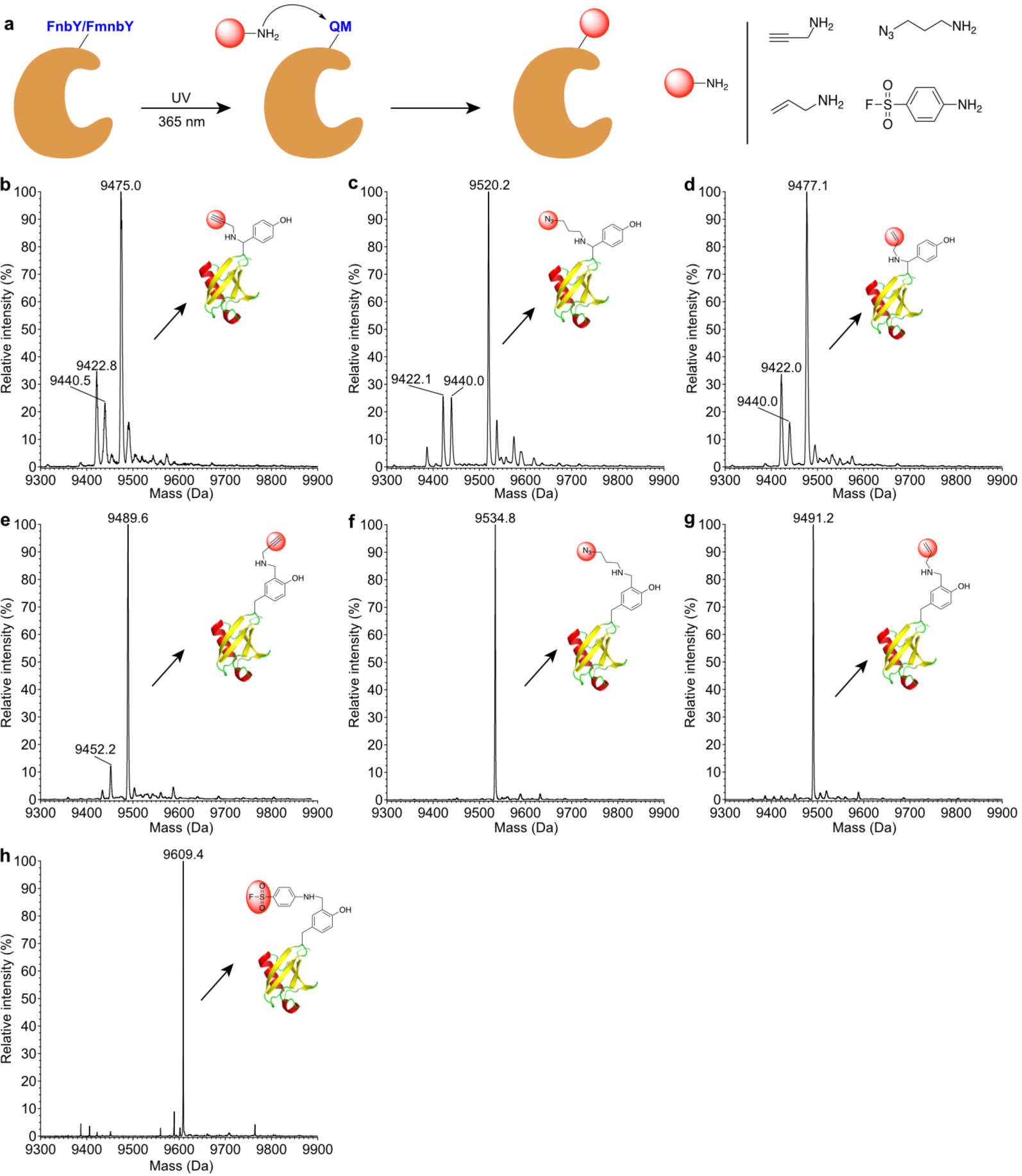
a, Scheme showing the conjugation procedure. b, Mass spectrum of Ub(6FnbY) conjugated with propargylamine. Expected, 9475.8 Da; measured 9475.0 Da. c, Mass spectrum of Ub(6FnbY) conjugated with 3-azido-1-propanamine. Expected, 9520.8 Da; measured 9520.2 Da. d, Mass spectrum of Ub(6FnbY) conjugated with allylamine. Expected, 9477.8 Da; measured 9477.1 Da. In spectra b-d, the peak observed at 9440 Da correspond to water hydrolysis of the photoactivated FnbY; the peak observed at 9422 Da correspond to F loss from the photoactivated FnbY, suggesting possible intramolecular Michael addition by nearby nucleophilic amino acid residue. e, Mass spectrum of Ub(6FmnbY) conjugated with propargylamine. Expected, 9489.8 Da; measured 9489.6 Da. f, Mass spectrum of Ub(6FmnbY) conjugated with 3-azido-1-propanamine. Expected, 9534.8 Da; measured 9534.8 Da. g, Mass spectrum of Ub(6FmnbY) conjugated with allylamine. Expected, 9491.8 Da; measured 9491.2 Da. h, Mass spectrum of Ub(6FmnbY) conjugated with 4-aminobenzene-1-sulfonyl fluoride. Expected, 9609.9 Da; measured 9609.4 Da.
