Extended Data Fig. 6. Pterin profile for the demonstration of DHP substrate origins.
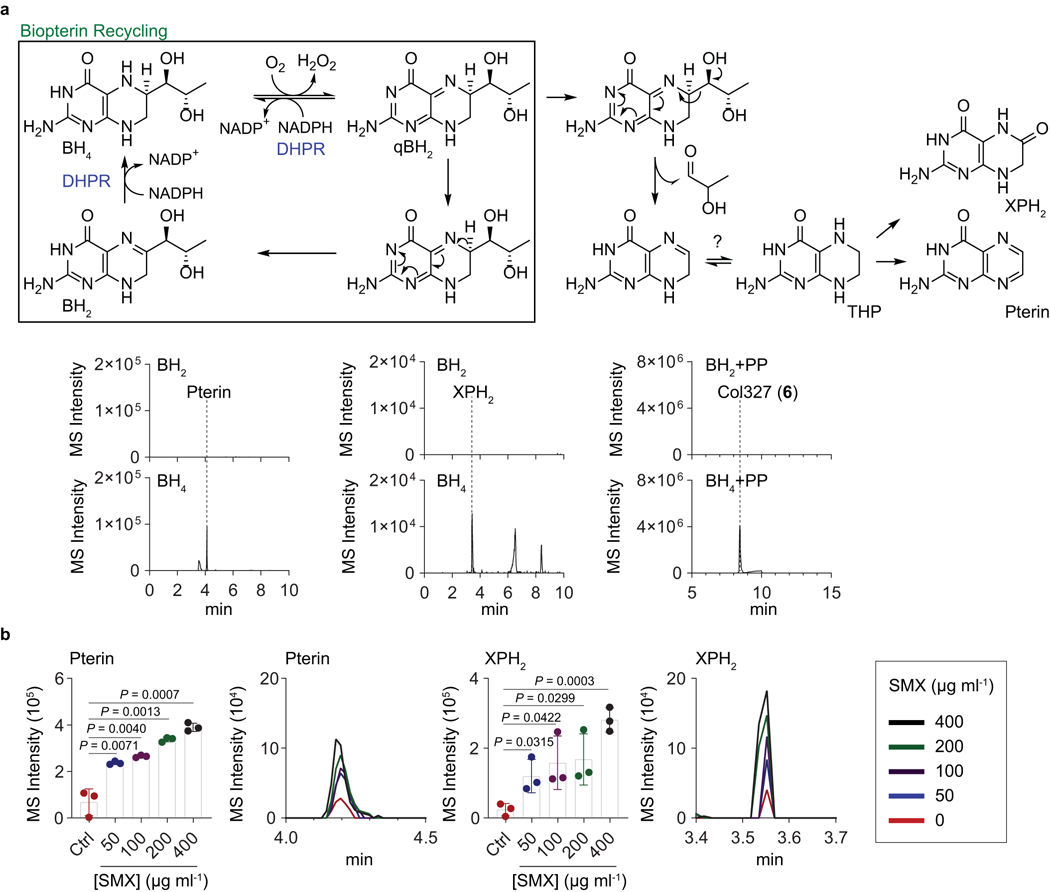
a, Previously described recycling and side-chain cleavage mechanism of biopterin in mammalian cells. Pterin and 7,8-dihydroxanthopterin (XPH2) are known as break-down products of biopterin. Similarly, we observed pterin (m/z 164.0572) and XPH2 (m/z 182.0678) in the BH4 in vitro chemical reaction via hydrogenation of commercial BH2 followed by aerobic incubation in water, not in the BH2 solution. Besides, colipterins were detected in BH4 solution supplemented with PP. Representatively, col327 (6) (m/z 328.1046) is shown. b, Upregulation of pterin and XPH2 in E. coli Nissle1917 in response to the sub-lethal levels of SMX. Dose-responses of pterin and XPH2 in antifolate SMX stress. The mean and s.d. (error bars) from three biological experiments (n = 3) are shown. Statistical significance was accessed using a two-tailed unpaired t-test. MS intensity of EIC chromatogram was determined by ion counts corresponding to pterin ([M+H]+ m/z 164.0572) and XPH2 ([M+H]+ m/z 182.0678) within a 10 ppm error window. High-resolution ESI-QTOF-LC-MS spectra of samples were analyzed using Phenomenex Kinetex C18 (100Å) 5 μm (250 × 4.6 mm) column with a gradient from 10%−100% aqueous acetonitrile in 0.1% formic acid over 30 min and with a 0.7 ml min−1 flow rate.
