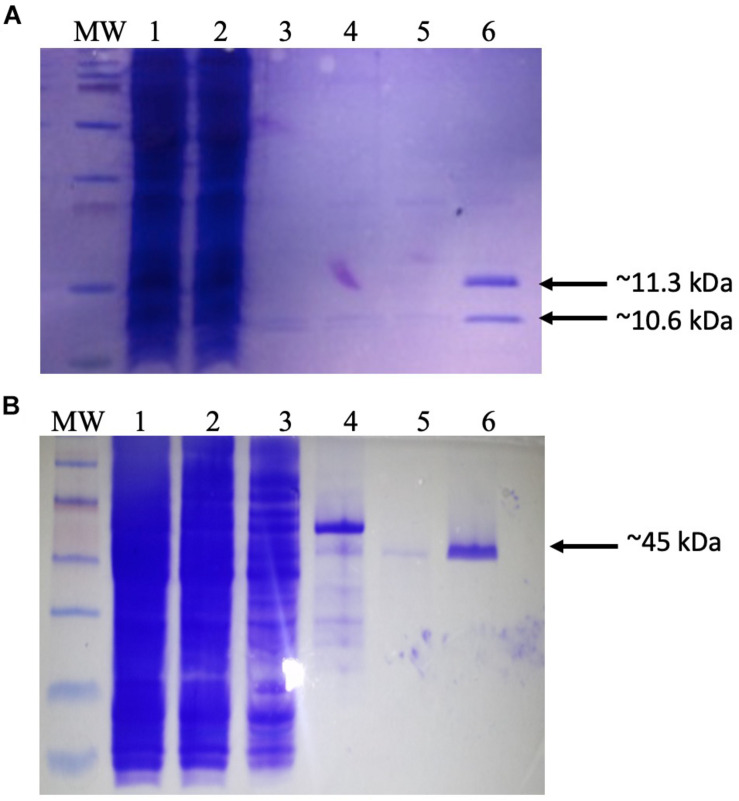
FIGURE 1.
Purification of recombinant proteins. (A) Production of dimeric integration host factor (IHF) from the construct pEE2003. Escherichia coli strain BL21 carrying the plasmid pEE203 (Supplementary Table 1) was cultured in 10 ml of Luria–Bertani (LB) broth, and overexpression was induced with 5 mM isopropyl β-d-1-thiogalactopyranoside. After 3–4 h, the cells were harvested, and the proteins were purified using a HisTrap (1 ml) column (GE Healthcare Life Sciences). The various steps of this process were analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Lanes: MW, prestained molecular weight markers (2–250 kDa) (Bio-Rad); 1, whole-cell lysate; 2, Ni column flow-through; 3–5, column washes; 6, IHF in 60 mM imidazole washing buffer. Arrows indicate the 11.3 kDa his-tagged IhfA subunit and the co-purifying 10.6-kDa IhfB subunit. (B) Production and purification of recombinant IntΦ24B. E. coli strain MC1061 carrying the plasmid pΦ24B-int (Supplementary Table 1) was cultured in 10 ml of LB broth, and overexpression was induced with L-arabinose. After 3–4 h, the cells were harvested, and the proteins were purified using a HisTrap (1 ml) column (GE Healthcare Life Sciences). The various steps of this process were analyzed on SDS-PAGE gel. Lanes: MW, pre-stained molecular weight markers (10–250 kDa; Bio-Rad); 1, whole-cell lysate; 2, Ni column flow-through; 3–5, column washes; 6, integrase in 60 mM imidazole. The arrow indicates the purified 45 kDa monomer of IntΦ24B.