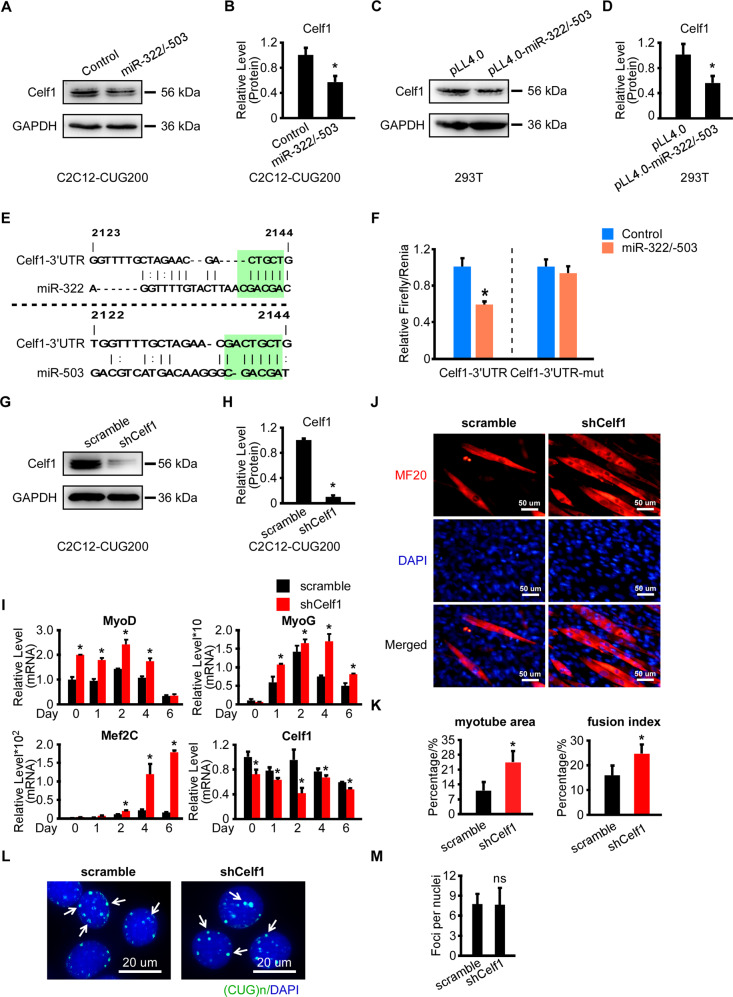
Fig. 4. miR-322/-503 targeted Celf1 leading to partial rescue of myoblast differentiation defects but not ribonuclear foci in DM1.
A, B Celf1 protein level was repressed by miR-322/-503 overexpression (OE) in C2C12-CUG200 cells. C, D Celf1 protein level was repressed by miR-322/-503 OE in HEK293T cells. E A mutual binding site of miR-322/-503 on Celf1-3′UTR was predicted by RNA22. F miR-322/-503 significantly repressed the relative luciferase activity of luciferase reporter that contained wild-type Celf1-3′UTR. However, the repression was abolished if the predicted binding sites of miR-322/-503 was mutated (Celf1-3′UTR-mut). G, H The knockdown of Celf1 in C2C12-CUG200 cells was verified by western blot. I Celf1 knockdown significantly upregulated the expressions of myogenic markers (MyoD, MyoG, and Mef2C) and downregulated Celf1 expression during C2C12-CUG200 differentiation. All expression levels were normalized to the control group at day 0. J Celf1 knockdown promoted myotube formation during C2C12-CUG200 differentiation. Myotube formation was displayed by immunostaining of MF20 on day 6. K Celf1 knockdown increased myotube areas (left) and fusion index (right) of C2C12-CUG200 differentiation. L, M Celf1 knockdown did not repress ribonuclear foci formation in C212-CUG200 cells. The ribonuclear foci were detected with CAG probe RNA FISH. Control, pLL4.0 vector stable transfection; miR-322/-503, pLL4.0-miR-322/-503 stable transfection; pLL4.0, pLL4.0 vector transient transfection; pLL4.0-miR-322/-503, pLL4.0-miR-322/-503 transient transfection; scramble, C2C12-CUG200/scramble cells; shCelf1, C2C12-CUG200/shCelf1 cells; * statistically significant (p < 0.05); ns not statistically significant.