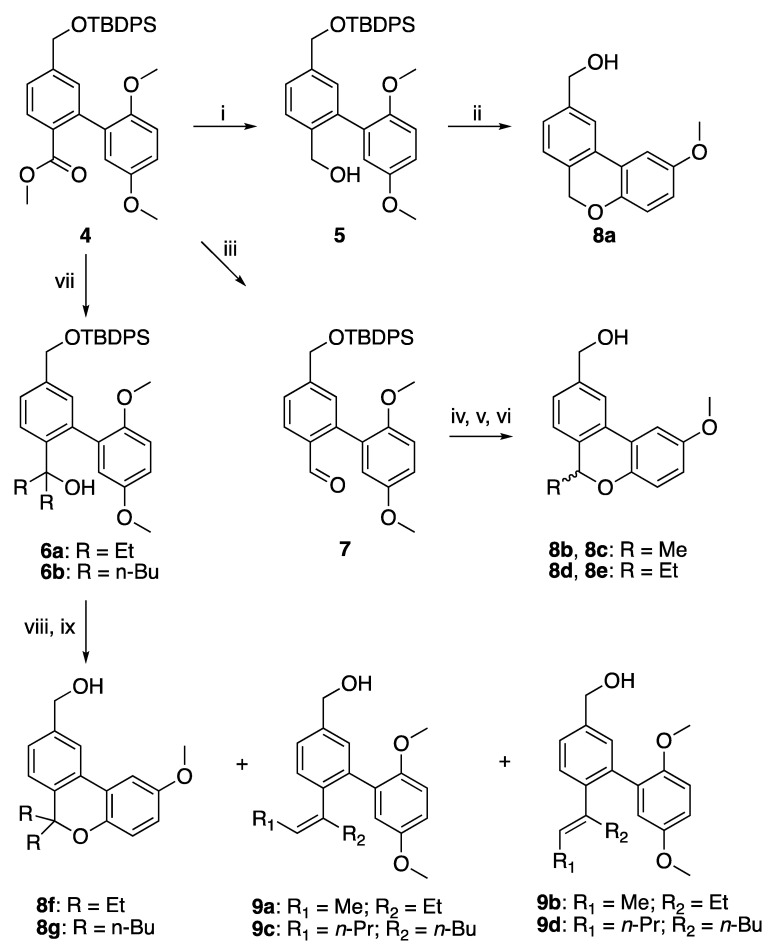
Scheme 1.
Reagents and conditions: (i) 4 (1 equiv), DIBALH (2.4 equiv), dry toluene, −78 °C; (ii) 5 (1 equiv), NaSEt (4 equiv), dry DMF, 110 °C; (iii) 4 (1 equiv), morpholine (2 equiv), DIBALH (1 equiv), dry THF, 0 °C; (iv) 7 (1 equiv), corresponding organolithium reagent (2 equiv), dry THF, 0 °C or −78 °C depending on the organolithium reagent; (v) PBr3 (0.34 equiv), LiI (3 equiv), dry CH2Cl2, rt; (vi) TBAF (2 equiv), THF, rt; (vii) 4 (1 equiv), organolithium reagent (4 equiv), dry THF, 0 °C or −78 °C depending on the organolithium reagent; (viii) 6 (1 equiv), HI (10 equiv), MeCN, rt; (ix) TBAF (1.1 equiv), THF, rt.