Figure 2.
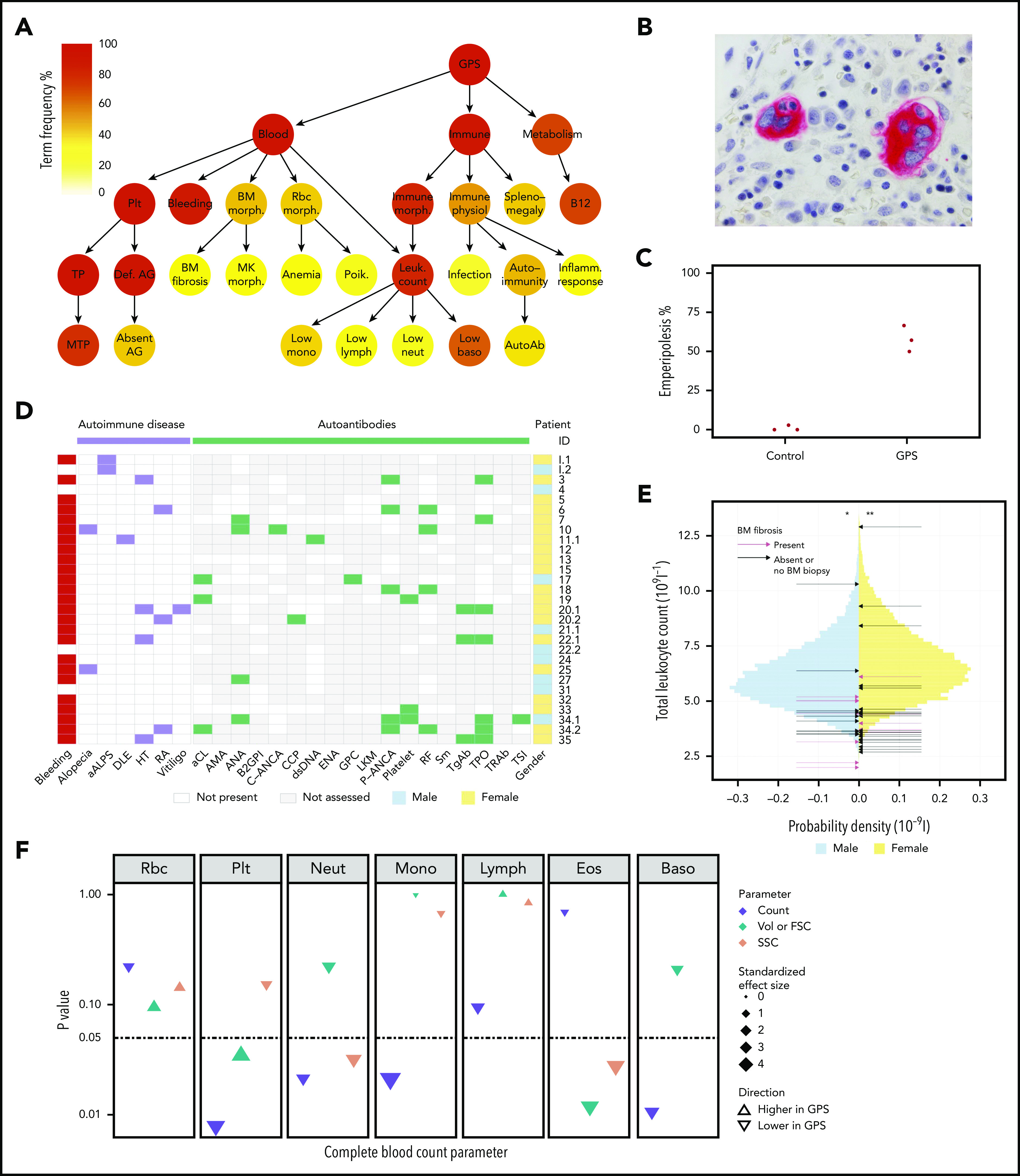
Novel clinical phenotypes. (A) Summary HPO tree, showing the 3 most frequent HPO organ systems represented in the 47 patients with GPS: Blood (“Abnormality of blood and blood-forming tissues”), Immune (“Abnormality of the immune system”), and Metabolism (“Abnormality of metabolism/homeostasis”). HPO terms affecting ≥8 patients are shown except terms associated with “Bleeding,” which are displayed in supplemental Figure 2.1. HPO term labels: Plt, “Abnormal thrombocyte morphology”; Bleeding, “Abnormal bleeding”; BM morph., “Abnormality of bone marrow cell morphology”; Rbc morph., “Abnormal erythrocyte morphology”; Immune morph., “Abnormal immune system morphology”; Immune physiol., “Abnormality of immune system physiology”; Splenomegaly, “Splenomegaly”; B12, “Abnormal vitamin B12 level”; TP, “Thrombocytopenia”; Def. AG, “Abnormal number of alpha granules”; BM fibrosis, “Myelofibrosis”; MK morph., “Abnormal megakaryocyte morphology”; Anemia, “Anemia”; Poik., “Poikilocytosis”; Leuk. count, “Abnormal leukocyte count”; Infection, “Recurrent infections”; Autoimmunity, “Autoimmunity”; Inflamm. response, “Increased inflammatory response”; MTP, “Macrothrombocytopenia”; Absent AG, “Absence of alpha granules”; Low mono, “Monocytopenia”; Low lymph, “Lymphopenia”; Low neut, “Neutropenia”; Low baso, “Decreased basophil count”; AutoAb, “Autoimmune antibody positivity.” (B) BM trephine image with CD61 stain (1000×) of patient ID 20.3, which shows neutrophil emperipolesis by 2 MKs. (C) Dot plot showing percentage of MKs with neutrophil emperipolesis in 3 patients with GPS (patient IDs 18, 33, and 34.1) (supplemental Figure 2.2 presents representative BM trephine images) and 3 control subjects. (D) Representation of autoimmune disease, results of autoantibody tests, and presence of bleeding symptoms in 29 patients with GPS (labeled according to patient ID) in whom autoantibody tests were performed. Autoantibodies tested in at least 3 patients are included (listed per order in graphic): aALPS, atypical autoimmune lymphoproliferative syndrome; DLE, discoid lupus erythematosus; HT, Hashimoto’s thyroiditis; RA, rheumatoid arthritis; and autoantibodies against: aCL, cardiolipin; AMA, mitochondria; ANA, nuclear; B2GPI, beta2-glycoprotein I; C-ANCA, neutrophil cytoplasmic; CCP, cyclic citrullinated peptide; dsDNA, double-stranded DNA; ENA, extractable nuclear antigen; GPC, gastric parietal cell; LKM, liver–kidney microsome; P-ANCA, neutrophil perinuclear; RF, rheumatoid factor; Sm, spliceosomal; TgAb, thyroglobulin; TPO, thyroperoxidase; TRAb, thyroid-stimulating hormone receptor; TSI, thyroid stimulating immunoglobulin. (E) Histogram showing the total leukocyte count of 45 032 blood donors in the INTERVAL study43 stratified according to sex, upon which the results of the patients with GPS are represented by arrows. The median total leukocyte count of both male and female patients with GPS was significantly (P = 3 × 10−3 and 4 × 10−4, respectively) lower than INTERVAL participants using a one-sample Wilcoxon signed-rank test, as represented by * and **. (F) CBC results for 5 patients with GPS vs 5 control subjects. The data point for each cell type and CBC parameter shows the absolute standardized effect size and directionality. On the y-axis (log10 scale), the P value (Mann-Whitney U test) is shown; the horizontal dotted line represents P < .05. Volume (Vol) or forward scatter (FSC) refers to the following measurements: mean cell volume (MCV) for red blood cells (Rbc), platelet mean frequent volume (P-MFV) for platelets (Plt); FSC for neutrophils (Neut), monocytes (Mono), lymphocytes (Lymph), eosinophils (Eos), and basophils (Baso). SSC was available for all parameters except basophils and reflects the complexity of cellular contents, including granularity.
