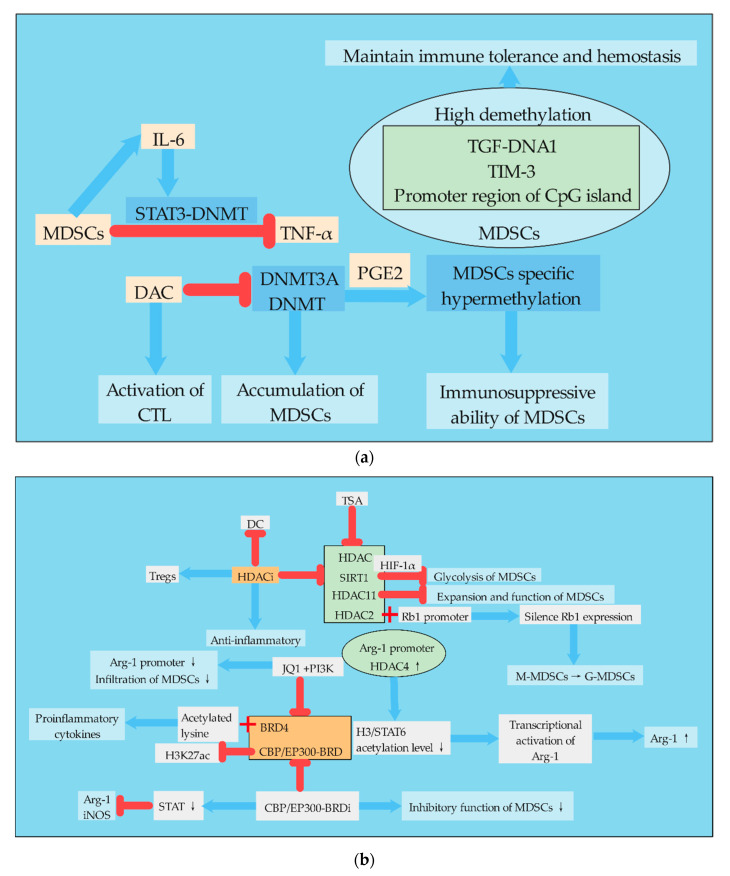
Figure 3.
Regulation of epigenetic modification in MDSCs. (a). MDSCs can activate the STAT3-DNMT epigenetic axis through autocrine IL-6 to inhibit TNF-α and promote tumor development. High demethylation of TGF-DNA1/TIM-3/CpG island promoter region in MDSCs is of great significance for the maintenance of immune tolerance and hemostasis. DAC can inhibit DNMT and thus the accumulation of MDSCs. DCA can also activate CTL directly. In the presence of PGE2, DNMT3A can promote MDSC-specific hypermethylation. (b). Histone acetylation plays an important role in the amplification of MDSCs. The regulation of MDSCs by histone acetylation is mainly realized by the dynamic balance between HATs and HDAC. TSA and HDACi inhibit HDAC. Moreover, HDACi can inhibit DCs and increase the number of Tregs, which has anti-inflammatory effects. HDAC11 can inhibit the amplification and function of MDSCs. The binding of HDAC2 and Rb1 promoter leads to Rb1 silencing and promotes the differentiation of M-MDSCs into G-MDSCs. Arg-1 is a key inhibitory molecule in MDSCs. The increase in HDAC4 in the promoter region of Arg-1 can lead to the decrease in acetylation level of H3/STAT6, thus promoting the transcriptional activation of Arg-1. BRD4 and CBP/EP300-BRD can inhibit histone acetylation and promote the production of proinflammatory factors. CBP/EP300-BRDi can inhibit CBP/EP300-BRD, leading to a decline in the inhibitory function of MDSCs.