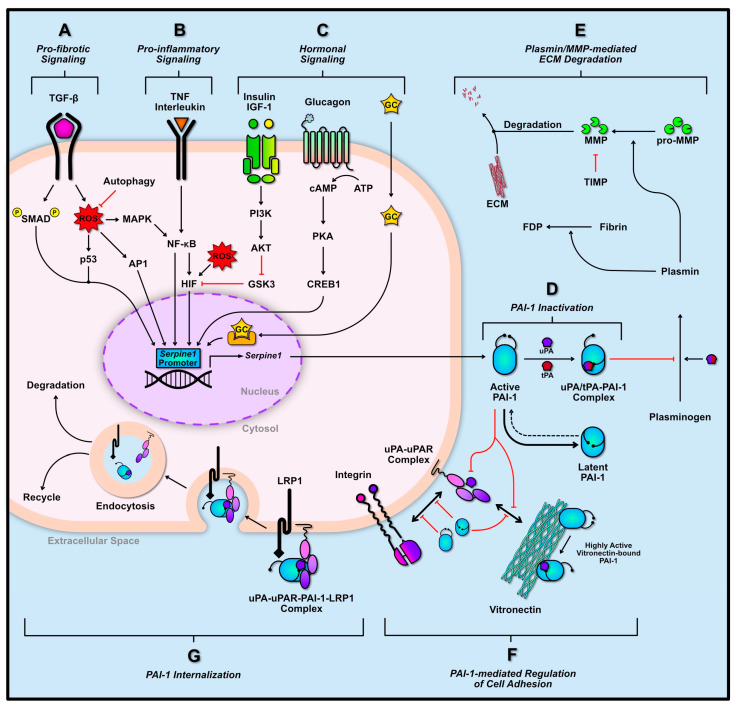
Figure 2.
Serpine1 transcriptional regulation and PAI-1 function. Serpine1 can be transcribed through several signaling cascades including pro-fibrogenic (A), pro-inflammatory (B), and pro-growth/hormonal signaling cascades (C). Once transcribed, PAI-1 is secreted in its active form into the extracellular space where it can inhibit urokinase-type PA (uPA)/tissue-type PA (tPA), and thus inhibit downstream extracellular matrix (ECM) degradation by preventing matrix metalloproteinase (MMP) activation (D,E). Conversely, PAI-1 may be rapidly converted to its more stable latent state. The active and latent PAI-1 molecules can interact with uPA/uPA receptor (uPAR) and integrins to diminish cell adhesion to vitronectin (F). Vitronectin-bound PAI-1 prevents its premature conversion to its latent state and improves its binding affinity to uPA/tPA. PAI-1 may also be internalized by the cell, through its interaction with lipoprotein receptor-related protein 1 (LRP1) and uPA/uPAR, ultimately leading to its degradation or recycling (G). Solid black arrows indicate activation. Dotted black lines indicate potential yet unfavorable pathways. Red bars indicate inhibition or blockage. Two-way arrows indicate interaction between proteins.