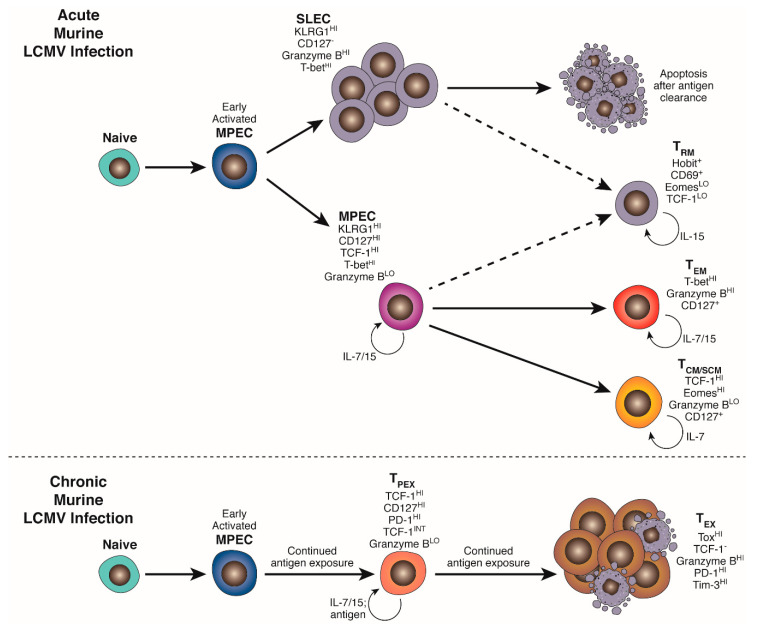
Figure 1.
Acute and chronic infection drive distinct programs of CD8+ T cell differentiation. Activated naïve CD8+ cells initiate a program of metabolic, transcriptional and epigenetic changes that facilitate differentiation into KLRG1HI CD127neg effector and KLRG1neg CD127HI memory precursors (MPEC). In an acute infection, an expanded pool of terminally differentiated cytotoxic effectors (SLEC/TEFF) clear infected cells and subsequently contract, leaving behind a heterogeneous pool of MPEC-derived self-renewing stem-like (TSCM) and central memory (TCM) in secondary lymphoid organs and effector memory (TEM) and resident memory (TRM) in peripheral tissues to provide protection against secondary exposure to the same pathogen. Chronic TCR stimulation, exacerbated by the absence of appropriate CD4+ T cell “help” and co-stimulatory and cytokine signalling, drives an alternative program of differentiation and epigenetic remodeling mediated by NFATc1, BATF, IRF4 and Tox. This gives rise to a TCF-1+PD-1INT pool of self-renewing “precursor” exhausted cells carrying a distinct epigenetic signature (TPEX) that produce and continually replenish an expanded pool of terminally exhausted progeny (TEX) able to partially control, but not fully clear, established infection. TEX maintain the epigenetic signature established in TPEX and exhibit a spectrum of effector function impairment and high expression of a suite of co-inhibitory receptors.