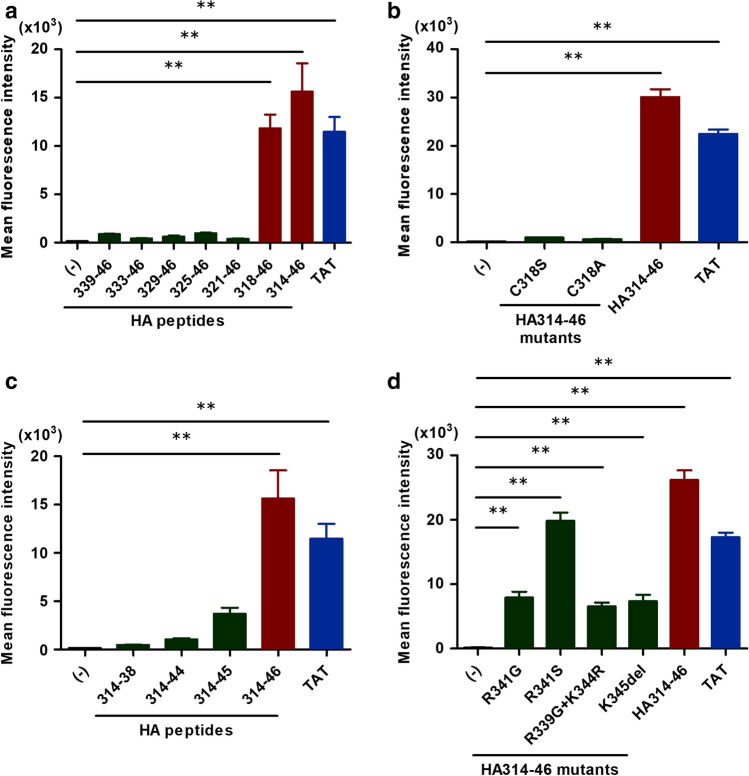
Figure 3.
Cysteine residue and/or multiple basic amino acid mutations impair the cell-penetrating activity of HA314-46 peptide. (a) Screening of membrane-permeable HA peptide sequences. KU812 cells were incubated with different lengths of HA peptides or a TAT peptide. (b) Cell-penetrating activities of cysteine-substitution mutants of HA314-46 peptide. KU812 cells were incubated with cysteine-substitution mutants of HA314-46 (C318S or C318A), HA314-46, or TAT peptide. (c) Cell-penetrating activities of C-terminal basic amino acid deletion mutants of HA314-46 peptide. KU812 cells were incubated with HA314-38, HA314-44, HA314-45, HA314-46, or TAT peptides. (d) Cell-penetrating activities of C-terminal amino acid mutants observed in naturally occurring isolates of H5 subtype. KU812 cells were incubated with intact or mutant HA314-46 or TAT peptide. After incubation with 10 μg/mL of FITC-conjugated peptides at 37 °C for 60 min, the cells were subjected to flow cytometric analysis to measure MFI of FITC. Data are presented as mean + SEM (n = 3 or 4). Asterisks indicate significant increase by one-way ANOVA with Bonferroni's multiple comparison test. **p < 0.01.