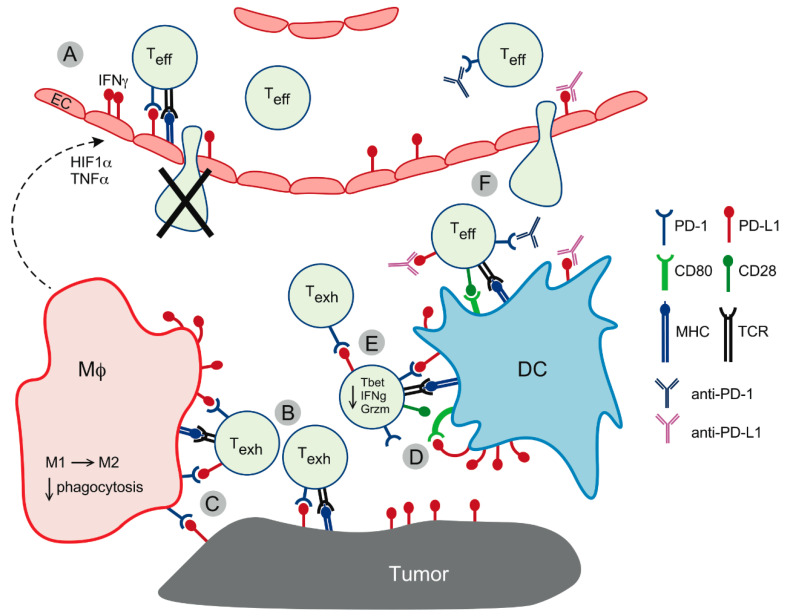
Figure 2.
PD-L1-mediated cellular interactions in the tumor microenvironment. (A) PD-L1 upregulation on blood vessel endothelial cells (EC) in response to T-cell-derived IFNγ and macrophage-derived hypoxia-inducible factor 1α (HIF1α) and tumor necrosis factor α (TNFα) functionally inactivates T-cells and reduces their transmigration into the tumor bed. Endothelial cells can also induce Fas-dependent T-cell death in migrating T-cells. (B) PD-L1 interacts with PD-1 on T-cells maintaining a state of exhaustion/dysfunction (Texh). (C) PD-L1 expressed on T-cells interacts with PD-1-positive macrophages (Mϕ), promoting M2 polarization and functional impairment. (D) PD-L1 on dendritic cells (DC) sequesters CD80 in cis, preventing it from interacting with CD28 on T-cells and thus abolishing T-cell activation. Excess of PD-L1 binds PD-1, contributing to T-cell exhaustion. (E) Reverse signaling via PD-L1 on T-cells impairs effector functions, such as cytokine production and killing capacity, while at the same time protecting T-cells from death, thus contributing to the expansion of functionally impaired T-cell clones. (F) Therapeutic antibodies restore T-cell effector function (Teff) by blocking PD-1 and/or PD-L1 signaling to the T-cell and releasing PD-L1-bound CD80 for interaction with CD28, thus enhancing T-cell stimulation upon antigen recognition via the T-cell receptor (TCR). In addition, therapeutic antibodies improve T-cell recruitment to the tumor by blocking PD-L1 on endothelial cells.