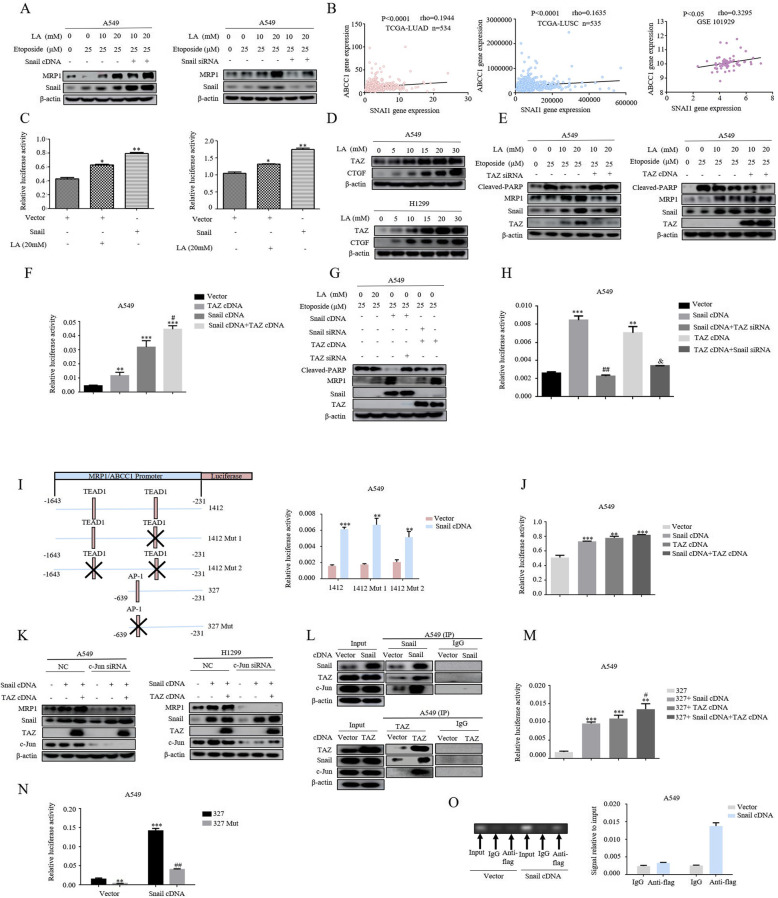
Fig. 4.
Formation of Snail/TAZ/AP-1 complex induced by lactate is required for MRP1 expression. a Transfection of A549 with either Snail cDNA or Snail siRNAs or their respective controls for 48 h, and then were treated first with LA for 3 h and then etoposide for additional 36 h before western blotting. b The correlations between SNAI1 and MRP1 expression from three data sets (TCGA-LUAD, TCGA-LUSC, GEO101929). The Spearman’s rank correlation coefficient (rho) and the P values were calculated. c A549 and H1299 cells were co-transfected with a 8xGTIIC-luc plasmid containing eight synthetic responsive elements of TEAD and Snail cDNA. Then luciferase activity was measured in the presence and absence of LA treatment and normalized using the dual luciferase reporter system. The bars represent the mean ± S.D. of triplicates. (*p<0.05, **P<0.01, for difference from untreated control by ANOVA for multiple comparison, ns means no statistical difference). d Western blot demonstrates increased TAZ and CTGF expression following 3 h of lactate (0, 5, 10, 15, 20 and 30 mM) stimulation in A549 and H1299 cells. e Transfection of A549 with either TAZ siRNA or TAZ cDNA or their respective controls for 48 h, and then were treated first with LA for 3 h and then etoposide for additional 36 h before western blotting. The results showed that MRP1 was significantly reduced after knockdown of TAZ and increased upon overexpression of TAZ. f Cells were co-transfected with MRP1 promoter reporter construct with a Snail cDNA construct, TAZ cDNA construct and combination of Snail cDNA construct and TAZ cDNA construct for 48 h before luciferase activity was determined and normalized. Data are represented as mean ± S.D. (*p < 0.05, **p < 0.01, ***p < 0.001, for difference from cells transfected with control vector, #p<0.05 for difference from Snail cDNA-transfected cells by ANOVA with Dunnett’s correction for multiple comparisons). g Western blot analysis of MRP1 expression after co-transfection of cells with Snail cDNA or siRNA or TAZ cDNA or siRNA for 48 h upon treatment described in Fig. 4e. h Cells were co-transfected with MRP1/ABCC1 promoter reporter construct with a wild-type Snail cDNA or TAZ cDNA or the combined Snail cDNA and TAZ siRNA or the combination of TAZ cDNA and Snail siRNA for 48 h before luciferase activity was determined and normalized. Data are represented as mean ± S.D. (*p < 0.05, ***p < 0.001, for difference from the untreated cells; ####p<0.001 for difference from cells transfected with Snail cDNA; &p < 0.05 for difference from cells transfected with TAZ cDNA by ANOVA with Dunnett’s correction for multiple comparisons). i A schematic representation of the MRP1/ABBC1 promoter region showing potential TEAD1 and AP-1 binding sites (left panel). Cells were co-transfected with one of these designated constructs (constructs 1412, construct 1412 Mut1 with one mutated TEAD1 binding site,1412 Mut2 with mutated two TEAD1 binding sites) and with a wild-type Snail cDNA construct for 48 h, and then luciferase activity was measured and normalized (right panel). The bars represent the mean ± S.D. of triplicates (**p < 0.01, ***p < 0.001, for difference from cells transfected empty vector by ANOVA with Dunnett’s correction for multiple comparisons). j The cells were co-transfected with the AP-1-luc plasmids containing the synthetic response element of AP-1(Jun:Jun; Jun:Fos) and the Snail cDNA or TAZ cDNA plasmids. Luciferase activity was then measured and normalized using a dual luciferase reporter system. Bars represent the mean ± S.D. Triplicate (**p < 0.01, ***p < 0.001, for difference from the cells transfected with control vector by ANOVA with Dunnett’s correction for multiple comparisons). k A549 and H1299 were co-transfected with c-JUN siRNA and Snail or TAZ cDNA constructs for 48 h. The blots have been probed with indicated antibodies. l A549 cells were transfected either with Snail cDNA or TAZ cDNA for 48 h. Cell lysates were subjected to immunoprecipitation with anti-Snail (upper panel) or with anti-TAZ (lower panel), immunoprecipitates were run in Western blots for Snail, TAZ and c-Jun. m Cells were co-transfected with MRP1 promoter reporter construct 327 with the Snail cDNA or TAZ cDNA plasmids for 48 h before luciferase activity was determined and normalized. Data are represented as mean ± S.D. (***p < 0.001, for difference from the cells transfected with control vector; #p < 0.05for difference from cells transfected with Snail cDNA by ANOVA with Dunnett’s correction for multiple comparisons). n Cells were co-transfected with one of two designated constructs (construct 327, 327 Mut with the mutated AP-1 binding site) and Snail cDNA plasmids for 48 h, and then luciferase activity was measured and normalized. The bars represent the mean ± S.D. of triplicates (**p < 0.01, ***p < 0.001, for difference from the cells transfected with construct 327 cells, ##p<0.01 for difference from 327Mut-transfected cells by ANOVA with Dunnett’s correction for multiple comparisons). o A549 cells were transfected with Flag-tagged Snail cDNA for 72 h. ChIP assays were performed using anti-FLAG antibody. The Standard PCR products were run and scanned (left panel). The histogram was presented as quantification of the PCR results (right panel)