Abstract
Background: Immune checkpoint inhibitors (ICIs) frequently cause thyroid dysfunction but their underlying mechanism remains unclear. We have previously demonstrated increased circulating natural killer (NK) cells and human leukocyte antigen (HLA)-DR surface expression on inflammatory intermediate CD14+CD16+ monocytes in programmed cell death protein-1 (PD-1) inhibitor-treated patients. This study characterizes intrathyroidal and circulating immune cells and class II HLA in ICI-induced thyroiditis.
Methods: This is a single-center prospective cohort study of 10 patients with ICI-induced thyroiditis by flow cytometry of thyroid fine needle aspirates (n = 9) and peripheral blood (n = 7) as compared with healthy thyroid samples (n = 5) and healthy volunteer blood samples (n = 44); HLA class II was tested in n = 9.
Results: ICI-induced thyroiditis samples demonstrated overall increased T lymphocytes (61.3% vs. 20.1%, p = 0.00006), CD4−CD8− T lymphocytes (1.9% vs. 0.7%, p = 0.006), and, as a percent of T lymphocytes, increased CD8+T lymphocytes (38.6% vs. 25.7%; p = 0.0259) as compared with healthy thyroid samples. PD-1 inhibitor-induced thyroiditis had increased CD4+PD1+ T lymphocytes (40.4% vs. 0.8%; p = 0.021) and CD8+PD1+ T lymphocytes (28.8% vs. 1.5%; p = 0.038) in the thyroid compared with the blood. Circulating NK cells, certain T lymphocytes (CD4+CD8+, CD4−CD8− T, gamma–delta), and intermediate monocytes were increased in ICI-induced thyroiditis. Six patients typed as HLA-DR4-DR53 and three as HLA-DR15.
Conclusions: ICI-induced thyroiditis is a T lymphocyte-mediated process with intra-thyroidal predominance of CD8+ and CD4−CD8− T lymphocytes. The HLA haplotypes may be involved but need further evaluation. These findings expand the limited understanding of ICI-induced thyroiditis, which could be further translated to guide immunomodulatory therapies for advanced thyroid cancer.
Keywords: thyroid immune-related adverse events, thyroiditis, thyroid dysfunction, hypothyroidism, autoimmunity
Introduction
Thyroid dysfunction is the most common endocrine immune-related adverse event (IRAE) of immune checkpoint inhibitor (ICI) immunotherapy. Thyroid IRAEs are more frequent with inhibitors of programmed cell death protein-1 (PD-1) or its ligand (PD-L1) at a rate of 7–21% (1–7) as compared with 0–6% with the cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitor ipilimumab (3,5,6). Clinically, thyroid IRAEs present as either new-onset hypothyroidism or transient thyrotoxicosis, which is usually followed by progression to hypothyroidism or recovery to normal thyroid function (1,2,4,8,9), with rare cases of persistent thyrotoxicosis after CTLA-4 inhibitors (10,11).
Thyroid peroxidase (TPO) antibody, associated with Hashimoto's thyroiditis, has been implicated in ICI-induced thyroiditis; but studies are conflicting (1,4,8), suggesting that the inconsistent presence of TPO antibody may merely reflect increased thyroid antigen exposure rather than a causal relationship. Several hypotheses have been proposed that might contribute to the development of thyroid IRAE, including genetic susceptibilities linked to human leukocyte antigen (HLA) haplotypes commonly associated with autoimmune thyroid disorders, CTLA-4 or PD-1 polymorphisms, underlying thyroid autoimmune susceptibility, de-repressed T regulatory cell functions, direct CD 8+ T lymphocyte toxicity, and/or cytokine (interleukin-2, interferon-alpha) mediated thyroiditis (12–17). More recently, histology from 2 cases of thyroid IRAE has demonstrated abundant clusters of necrotic cells, lymphocytes, and histiocytic cells (18,19); however, these case studies did not further characterize the intra-thyroidal immune cell phenotype.
While collectively these findings support that ICIs, especially inhibitors of PD-1/PD-L1, cause immune-mediated thyroiditis, the precise underlying immune mechanisms have not been identified. We (20) and others (4) have shown that patients with thyroid IRAE have better overall survival, suggesting that development of this side effect may be a biomarker of response to ICI therapy in patients with advanced cancer. Elucidating the underlying mechanisms for ICI-induced thyroiditis is important not only for predicting the occurrence and severity of thyroiditis but also because this information could guide selection of immunomodulatory therapies for treatment of advanced, therapeutically refractory thyroid cancer patients.
We hypothesized that thyroid and peripheral blood immunophenotyping will identify cell types in ICI-induced thyroiditis; and examination of HLA haplotypes may help identify, as in other endocrinopathies, patients susceptible to thyroiditis. In this regard, we have previously demonstrated increased circulating CD56+CD16+ natural killer (NK) cells and elevated HLA-DR surface expression in the inflammatory intermediate CD14+CD16+ monocytes in anti-PD-1-treated patients (1). To our knowledge, immunophenotyping of thyroid fine needle aspirate (FNA) samples has not been performed in ICI-induced thyroid disorders. In this study, we aimed at identifying immune cells and HLA haplotypes associated with ICI-induced thyroiditis (referred to as thyroid IRAE), and build on our prior study with a goal of understanding how ICIs target the thyroid gland.
Materials and Methods
Subject identification and case definition
Cancer patients receiving PD-1 or PD-L1 inhibitors at Mayo Clinic, Rochester who developed thyroid dysfunction between May 1st, 2018 and June 30th, 2019 were enrolled in this Mayo Clinic Institutional Review Board (IRB) approved prospective cohort pilot study. Screening thyroid function tests (TFTs) consisting of thyrotropin (TSH), free thyroxine (fT4), and/or total triiodothyronine (T3) were performed in the majority of patients at baseline and in all patients every three to four weeks or before each ICI dose. Subjects, both cases and controls, were enrolled after an informed and voluntary consent process. ICI-induced thyroiditis was defined as previously published (1) when a patient had two or more abnormal TFTs after starting an ICI, and in the absence of other causes. Thyroid dysfunction was characterized as (a) primary hypothyroidism, either overt defined by TSH ≥4.3 mIU/L and fT4 ≤ 0.8 ng/dL or subclinical defined by TSH ≥4.3 mIU/L and fT4 0.9–1.7 ng/dL; and (b) thyrotoxicosis, either overt defined by TSH ≤0.2 mIU/L and fT4 ≥ 1.8 ng/dL or total T3 ≥ 200 ng/dL, or subclinical defined by TSH ≤0.2 mIU/L, fT4 0.9–1.7 ng/dL, and total T3 80–200 ng/dL. Demographic, clinical, biochemical, and radiologic data were collected, including prior treatment with other immunotherapies. When available, 18-fluorodeoxy glucose (18FDG)-positron emission tomography (PET) images were reviewed for the presence or absence of increased 18FDG uptake within the thyroid gland. All clinically indicated laboratory testing was performed at the Mayo Medical Laboratory, Rochester, Minnesota. Our laboratory's reference rages for adults are 0.3–4.2 mIU/L for TSH, 0.9–1.7 ng/dL for fT4, and 80–200 ng/dL for total T3. Results of TPO antibody (reference range <9.0 IU/mL), TSH receptor antibody (reference range ≤1.75 IU/L), thyroid-stimulating immunoglobulin (TSI) (reference range ≤1.3 TSI index), and thyroglobulin antibody (reference range <4.0 IU/mL) when tested were also collected.
Laboratory methods
Sample collection was performed within 2 weeks of abnormal TFTs in all except for 2 patients: 1 had sample collected 2 months later, and the other only had blood collected for HLA analysis. None of the patients experienced any adverse events from participating in the study.
Peripheral blood immunophenotyping
To characterize the circulating immune phenotype in ICI-induced thyroiditis, we performed flow cytometry on peripheral blood samples collected in K2EDTA tubes (Becton Dickinson, Franklin Lakes, NJ) from 6 patients (5 PD-1 and 1 PD-L1) in addition to 7 patients reported by Delivanis et al. (1). These profiles were compared with 44 healthy volunteers whose circulating immune phenotype has been previously reported (21). Un-manipulated whole blood was stained with antibodies directly. Flow cytometry was performed on the three-laser, 10-color Gallios Flow Cytometer (Beckman Coulter, Brea, CA). All procedures, antibodies, flow protocols, instrument settings, and gating strategies for peripheral blood flow cytometry have been previously described in prior publications by Gustafson et al. (22,23) and Delivanis et al. (1). Analysis of the flow cytometry data was performed by using Kaluza (Beckman Coulter) software, allowing quantification of the absolute number and percent of immune cell subtypes. Descriptive statistics are reported as mean and standard deviation, while categorical data are shown as number and percentage. Comparisons between different cohorts were tested for statistical significance via the Student's t-test by using a false discovery rate of 15%. All graphical representations and statistical analyses were performed in Prism 7 (GraphPad, San Diego, CA).
Thyroid FNA immunophenotyping
Thyroid FNA was performed with ultrasound guidance by using 27 gauge needles for a maximum of 4 passes to obtain a sufficient sample for flow cytometry. Samples were collected in K2EDTA tubes with saline suspension from 9 thyroiditis subjects (within 2 weeks of abnormal TFT in n = 8; 2 months after abnormal TFT in n = 1), and from surrounding healthy thyroid parenchyma in 5 subjects with benign thyroid nodules who underwent FNA. To identify leukocyte populations in the thyroid FNA sample, DuraClone IM Phenotyping Basic Tubes (Beckman Coulter, Indianapolis, IN) were used. Briefly, 10 μL of HLA-A, B, C PerCP (BioLegend, San Diego, CA) and 1 μL of LIVE/DEAD Fixable stain for 405 nm excitation (ThermoFisher Scientific, USA) were added to the DuraClone IM tube. Then, 100 μL of sample was added, blocked with 50 μL of mouse serum (Sigma-Aldrich, St. Louis, MO), vortexed, and incubated for 15 minutes at room temperature in the dark. The sample was lysed with 1 mL of Versa-Lyse lysing buffer (Beckman Coulter, Indianapolis, IN) for 30 minutes in the dark at room temperature. Flow-Count Fluorospheres (100 μL; Beckman Coulter) were added and immediately followed by sample analysis. CD45 and HLA-ABC were used to distinguish leukocytes (CD45+HLA-ABC+) from nonhematopoietic cells (CD45-HLA-ABC+) to enable the enumeration of cell populations. This was followed by T lymphocyte, B lymphocyte, and NK cell analysis; this was subsequently followed by a deeper analysis of T lymphocyte phenotypes. For thyroid FNA flow cytometry, we used the Duraclone IM tubes but gated the same way as previously published for peripheral blood (23), and analyzed as mentioned earlier.
HLA class II typing
Low- to medium-resolution Class II HLA typing at HLA-DRB1, DRB3/4/5, DQB1, and DQA1 loci was performed by the reverse sequence-specific oligonucleotide (r-SSO) method as per the manufacturer's protocol (LabTYPE, One Lambda, Canoga Park, CA).
Results
Patient characteristics
During the study period, we enrolled 10 patients with ICI-induced thyroiditis (8 after PD-1 inhibitor and 2 after PD-L1 inhibitor). The most common malignancy was melanoma, the median age was 61 years (range 39, 76), and 50% were females. Nine patients presented with thyrotoxicosis, of whom 7 progressed to overt hypothyroidism and 2 had normalization of thyroid function; and 1 presented with primary hypothyroidism. Other endocrine side effects occurred in 2 patients (diabetes mellitus in one and hypophysitis in the other). Detailed patient characteristics are presented in Table 1. Of these 10 patients, flow cytometry analysis was performed on thyroid FNA in 8 patients within 1 week of thyroiditis (compared with n = 5 healthy) and 1 patient 2 months later. Flow cytometry analysis was performed on peripheral blood in 7 patients. The MHC class II haplotype was tested in the peripheral blood of 9 patients.
Table 1.
Characteristics of Individual Patients with Thyroid Immune-Related Adverse Events
| Age/sex | Cancer | ICI | Time to onset (week) | Thyroid IRAE | Anti-TPO(IU/mL) | 18FDG-PETscan | Flow cytometry | MCH class II |
|---|---|---|---|---|---|---|---|---|
| 39/M | Melanoma | Nivolumab and ipilimumab | 6.43 | Overt thyrotoxicosis f/b overt primary and central hypothyroidism | 1.2 | Diffuse thyroid uptake | Thyroid only | DR52DR53DR4DR11DQ7DQ7 |
| 47/M | Melanoma | Pembrolizumab | 3.71 | Overt thyrotoxicosis f/b overt hypothyroidism | 3.3 | Not done | Thyroid and blood | DR53DR51DR4DR15DQ7DQ6 |
| 75/F | Merkel cell | Avelumab | 23.86 | Subclinical thyrotoxicosis f/b overt hypothyroidism | - | Diffuse thyroid uptake | Thyroid only | Not collected |
| 70/M | Bladder | Pembrolizumab | 2.86 | Overt thyrotoxicosis f/b overt hypothyroidism | >800 | Not done | Thyroida and blood | DR53DR53DR4DR9DQ7DQ9 |
| 51/F | Melanoma | Nivolumab and ipilimumab | 2.86 | Overt thyrotoxicosis f/b euthyroidism | 2.6 | Diffuse thyroid uptake | Thyroid and blood | DR51DR8DR15DQ4DQ6 |
| 62/F | Lung | Pembrolizumab | 4.29 | Overt thyrotoxicosis f/b euthyroidism | - | Not done | Thyroid and blood | DR52DR52DR17DR14DQ2DQ6 |
| 76/F | Endometrium | Nivolumab | 4.14 | Overt thyrotoxicosis f/b overt hypothyroidism | - | Not done | Thyroid and blood | DR53DR53DR4DR7DQ7DQ9 |
| 41/M | Melanoma | Pembrolizumab | 2.86 | Overt thyrotoxicosis f/b overt hypothyroidism | 277.2 | Not done | Thyroid and blood | DR51DR51DR15DR15DQ5DQ5 |
| 62/M | Melanoma | Nivolumab | 4.00 | Overt thyrotoxicosis f/b overt hypothyroidism | 10 | Diffuse thyroid uptake | Not done | DR53DR53DR4DR7DQ2DQ8 |
| 60/M | Lung | Durvalumab | 52.00 | Subclinical hypothyroidism f/b overt hypothyroidism | 0.6 | Not done | Thyroid and blood | DR52DR53DR17DR4DQ2DQ8 |
Flow cytometry sample collected two months after thyroiditis diagnosis.
FDG-PET, 18flouorodeoxyglucose-positron emission tomography; ICI, immune checkpoint inhibitor; IRAE, immune-related adverse event; MHC, major histocompatibility complex; TPO, thyroid peroxidase.
Thyroid FNA immunophenotyping
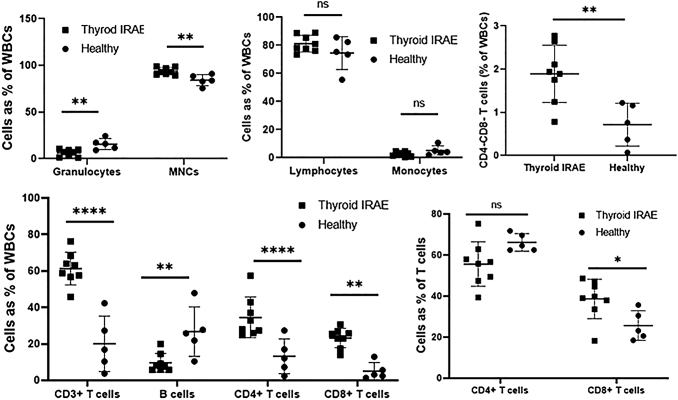
The dot plots comparing an ICI-induced thyroiditis patient and a healthy thyroid sample are presented in Figure 1A and B. The ICI-induced thyroiditis samples had 5.9% and healthy controls had 0.5% CD45+ white blood cells (WBCs) out of all cells. Of the CD45+ WBCs, ICI-induced thyroiditis had reduced granulocytes (6.2% vs. 15.7%, p = 0.004), increased mononuclear cells (93.8% vs. 84.3%, p = 0.004), and similar overall lymphocytes (81.2% vs. 74.5%, p = 0.19) as compared with healthy controls (Supplementary Table S1; Fig. 2). Within the lymphocyte compartment, we observed increased CD3+ T lymphocytes (61.3% vs. 20.1%, p = 0.00006) with reduced CD19+ B lymphocytes (9.6% vs. 26.7%, p = 0.007), increased CD4+ T lymphocytes (34.5% vs. 13.2%, p = 0.005) but much more CD8+ T lymphocytes (23.2% vs. 5.1%, p = 0.00006) (Supplementary Table S1; Fig. 2), increased CD4−CD8− T lymphocytes (1.9% vs. 0.7%, p = 0.006), and more but not statistically different NK cells, as compared with healthy controls (Supplementary Table S1). Within the T lymphocyte compartment, ICI-induced thyroiditis had increased CD8+T lymphocytes (38.6% vs. 25.7%; p = 0.026) with a corresponding but not statistically significant decrease in CD4+ T lymphocytes (55.6% vs. 66.2%; p = 0.063) as compared with healthy controls (Fig. 2).
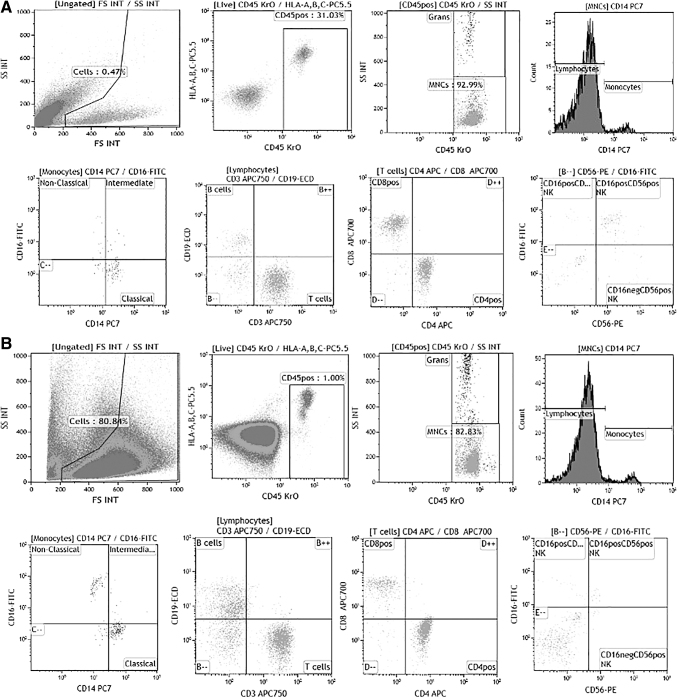
FIG. 1.
(A) Thyroid fine needle aspirate immunophenotyping dot plots in a patient with PD-1 inhibitor-induced thyroiditis. (B) Thyroid fine needle aspirate immunophenotyping dot plots in a volunteer with healthy thyroid. PD-1, programmed cell death protein-1.
FIG. 2.
Thyroid fine needle aspirate immunophenotyping data comparing thyroid IRAE samples collected within 2 weeks of diagnosis (n = 8) and healthy thyroid samples (n = 5). *p-value <0.05; **p-value <0.01; ***p-value <0.001; ****p-value <0.0001. IRAE, immune-related adverse event.
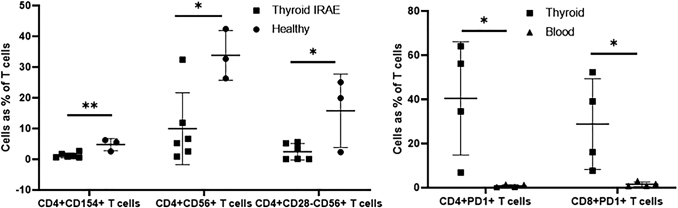
An in-depth analysis of T lymphocyte phenotypes was performed on thyroid FNA samples from 6 patients within 2 weeks of thyroiditis diagnosis (4 after PD-1 inhibitor and 2 after PD-L1 inhibitor) (Supplementary Table S2). As a percentage of CD3+ T lymphocytes, we observed less CD4+CD154+ (1.25% vs. 4.8%; p = 0.005), CD4+CD56+ (9.9% vs. 33.8%; p = 0.016), and CD4+CD28−CD56+ (2.4% vs. 15.8%; p = 0.027) T lymphocytes in ICI-induced thyroiditis as compared with controls (Supplementary Table S2; Fig. 3).
FIG. 3.
Thyroid fine needle aspirate further T cell phenotype analysis comparing thyroid IRAE samples collected within 2 weeks of diagnosis (n = 6) and healthy thyroid samples (n = 3) (left panel) and comparing thyroid and blood in PD-1 inhibitor-induced thyroiditis (n = 4) (right panel). *p-value <0.05; **p-value <0.01; ***p-value <0.001; ****p-value <0.0001.
Peripheral blood immunophenotyping
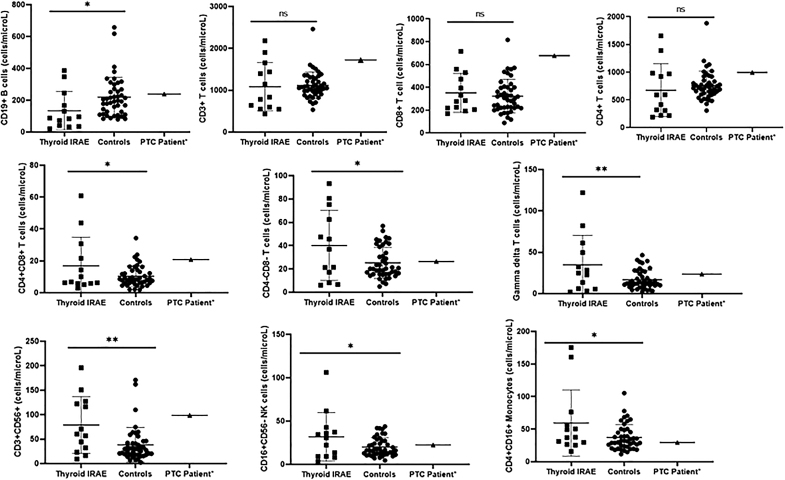
Peripheral blood immunophenotyping was performed on 6 patients, expanding on our previously published cohort of 7 patients with pembrolizumab-induced thyroiditis (1). This demonstrated fewer circulating CD19+ B lymphocytes (134.2 cells/μL vs. 218.8 cells/μL; p = 0.036) but increased circulating CD16+56− NK cells (31.9 cells/μL vs. 20.1 cells/μL; p = 0.024), CD4+CD8+ T lymphocytes (16.9 cells/μL vs. 10.4 cells/μL; p = 0.045), CD4−CD8− T lymphocytes (40.2 cells/μL vs. 25.2 cells/μL; p = 0.012), gamma–delta T lymphocytes (34.9 cells/μL vs. 16.9 cells/μL; p = 0.005), and intermediate monocytes (59.4 cells/μL vs. 37.3 cells/μL; p = 0.021) as compared with healthy volunteers (n = 44) (Supplementary Table S3; Fig. 4).
FIG. 4.
Peripheral blood immunophenotyping comparing 13 thyroid IRAE patients (n = 7 from a previous study and n = 6 from the current study), 44 healthy controls, and 1 patient with melanoma and papillary thyroid cancer who received pembrolizumab. *p-value <0.05; **p-value <0.01; ***p-value <0.001; ****p-value <0.0001.
Comparing thyroid and peripheral blood immunophenotyping in ICI-induced thyroiditis
ICI-induced thyroiditis patients (n = 8 samples collected within 2 weeks) had increased intra-thyroidal CD3+ and CD8+ T lymphocytes but not significantly different in blood (n = 13) as compared with healthy volunteers (n = 44). On the other hand, NK cells and intermediate monocytes were increased in the blood but not significantly different in the thyroid as compared with controls. B lymphocytes were less and CD4−CD8− T lymphocytes were more in both the thyroid and blood in ICI-induced thyroiditis patients as compared with controls.
An in-depth analysis of T lymphocyte phenotypes demonstrated that among ICI-induced thyroiditis patients, CD4+CD28+ (68.1% vs. 92.2%; p = 0.023) but not CD8+CD28+ T lymphocytes (64.7% vs. 83.1%; p = 0.14) were less in thyroid (n = 6) as compared with the blood (n = 6). PD-1 inhibitor-induced thyroiditis (n = 4) had more CD4+PD1+ (40.4% vs. 0.8%; p = 0.021) and CD8+PD1+ T lymphocytes (28.8% vs. 1.5%; p = 0.038) in the thyroid compared with being almost absent in the blood (Fig. 3). These patients also had significantly less CD4+PD1+ and CD8+PD1+ T lymphocytes in the blood as compared with healthy volunteers, similar to that previously reported by our laboratory (1). On the other hand, PD-L1 inhibitor-induced thyroiditis (n = 2 thyroid and n = 1 blood; statistical testing not feasible) demonstrated similar CD4+PD1+ and CD8+PD1+ T lymphocytes in the thyroid and blood, with blood values being much higher than those in PD-1 inhibitor-induced thyroiditis (Fig. 3).
Class II HLA types
Testing of Class II HLA was performed in peripheral blood from 9 thyroiditis cases, demonstrating that 6 typed as HLA-DR4-DR53 and 3 typed as HLA-DR15. Details for individual patients are given in Table 1.
Melanoma patient with papillary thyroid cancer
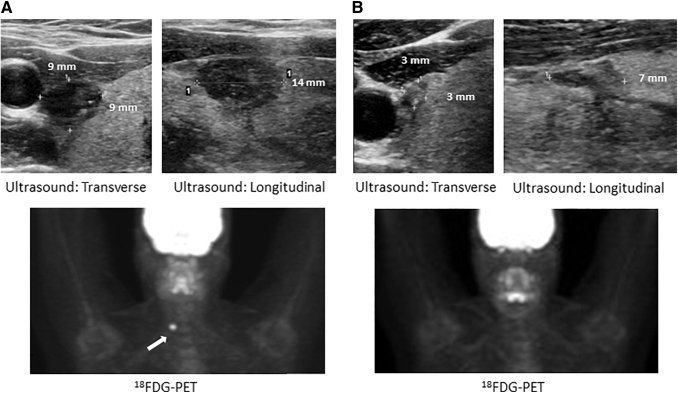
Included in this study is a 40-year-old female with a metastatic melanoma who on staging 18FDG-PET was found to have an incidental 18FDG avid thyroid nodule measuring 1.4 × 0.9 × 0.9 cm (volume 0.57 mL), diagnosed as papillary thyroid cancer (PTC) on FNA cytology, but was observed conservatively given her advanced melanoma. She was initiated on PD-1 inhibitor pembrolizumab every three weeks, which she continued for one year, achieving a complete response by response evaluation criteria in solid tumors. After 1 year of therapy, the PTC significantly regressed to a size of 0.7 × 0.3 × 0.3 cm (volume 0.03 mL), thus showing a 95% reduction in volume along with resolution of 18FDG uptake (Fig. 5A, B). This patient also developed hypophysitis manifesting as central hypothyroidism and secondary adrenal insufficiency. Statistical comparison was not feasible but this patient had more circulating overall T lymphocytes (1720.7 vs. 1116), specifically CD8+ (678 vs. 323.2) and CD4+CD8+ (20.8 vs. 10.4), as well as CD56+CD16+ NK cells (233 vs. 147.4) as compared with healthy volunteers (Supplementary Table S3; Fig. 4). Repeat FNA did not yield enough sample for cytology or immunophenotyping, consistent with regression of the nodule. HLA class II evaluation demonstrated DR53DR51DR7DR15DQ2DQ6.
FIG. 5.
Ultrasound and 18FDG-PET scan of patient with melanoma and papillary thyroid cancer (A) demonstrating regression in size and resolution of 18FDG avidity of papillary thyroid cancer one year after Pembrolizumab therapy for melanoma (B). 18FDG, 18-fluorodeoxy glucose; PET, positron emission tomography.
Discussion
In the present pilot study, PD-1/PD-L1 inhibitor-induced thyroiditis was associated with increased intra-thyroidal CD8+, PD1+, and CD4−CD8− T lymphocytes as compared with controls, suggesting their prominent role in the mechanism. We also observed increased circulating subtypes of T lymphocytes (CD4−CD8−, gamma–delta, CD4+CD8+), subtypes of NK cells, and intermediate monocytes, with a corresponding decrease in B lymphocytes. To our knowledge, this study is the first to comprehensively characterize the immune phenotype in both the thyroid and blood of patients with ICI-induced thyroiditis.
The preferential increase in T lymphocytes, specifically CD8+, in the thyroid but not in the blood of ICI-induced thyroiditis patients as compared with healthy controls could represent expansion of intra-thyroidal T lymphocytes or infiltration from circulating T lymphocytes. CD4−CD8− (double negative) T lymphocytes were increased in the blood and thyroid of patients with thyroiditis as compared with healthy volunteers. This cell population likely contains a large population of gamma–delta T lymphocytes that have been linked to potentiating Hashimoto's thyroiditis through increased antibody production (24). In addition, these self-reactive, pro-inflammatory effector cells have been shown to infiltrate inflamed tissues and contribute to organ damage (25). The decrease in all lymphocyte populations two months after ICI-induced thyroiditis in a patient suggests that the inflammatory process is reversible within the thyroid gland itself, which may predict biochemical recovery, but needs further investigation.
Increased circulating subtypes of NK cells, CD4+CD8+ T lymphocytes, gamma–delta T lymphocytes, and intermediate monocytes in thyroiditis patients as compared with healthy volunteers could be independent of the thyroiditis process, reflecting either cancer or ICI-induced changes. However, NK cells were increased in the thyroid of one patient who developed overt thyrotoxicosis followed by hypothyroidism and also hypophysitis; hence, it could be a marker for more severe and multiple endocrine IRAEs. The expression of CD4 and CD8 co-receptors on mature T cells is generally considered to be mutually exclusive; however, CD4+CD8+ T lymphocytes have been demonstrated in the target organ affected by several autoimmune conditions, including autoimmune thyroiditis (26), atopic dermatitis (27), systemic sclerosis (28), and rheumatoid arthritis (29), suggesting their role in these autoimmune conditions. Their increased numbers in blood of thyroiditis patients could suggest their role in ICI-induced thyroiditis as well. T lymphocytes expressing the gamma–delta form of the T cell receptor are a distinct functional class whose physiologic role is not clearly understood. In normal individuals, the great majority of these cells are double negative (CD4−CD8−), in fact reflecting a concordant intra-thyroidal increase in this population. Much remains to be learned regarding the physiologic role (s) of these cells but it has been postulated that they probably contribute more to immunoregulation and tissue repair than to immunoprotection (30). We postulate that lower intrathyroidal and circulating B lymphocytes in thyroiditis patients suggest a shift of the immune phenotype toward an increase in T lymphocytes with a corresponding decrease in B lymphocytes.
Further, T lymphocyte immunophenotyping was consistent with the preferential intra-thyroidal increase in CD8+ as compared with CD4+ T lymphocytes in ICI-induced thyroiditis. PD-1 inhibitor-induced thyroiditis had more intra-thyroidal CD4+PD1+ and CD8+PD1+ T lymphocytes as compared with being almost absent in the blood; whereas PD-L1 inhibitor-induced thyroiditis had similar numbers of these cells in thyroid and blood, with circulating values being much higher than those in PD-1 inhibitor-induced thyroiditis. As previously reported by our lab (1), circulating PD-1+ T lymphocytes in PD-1 inhibitor-treated patients were almost absent, indicating either loss or impaired detection of these cell types. Unexpectedly, we did observe intra-thyroidal PD1+ T lymphocytes in thyroiditis patients, which could represent resident thyroid T lymphocytes not blocked by the PD-1 inhibitor or perhaps related to assay methodology. In limited prior studies, expression of PD-L1 is low to absent in normal thyroid, but it is increased in Hashimoto's thyroiditis and thyroid cancer (31,32). Thus, the presence or absence of PD-L1 with intra-thyroidal PD1+ T lymphocytes may impact susceptibility to thyroiditis from ICIs.
HLA class II types DR3 and DR4 have been reported to be associated with autoimmune thyroid disorders, Graves' disease (33,34) more frequently than Hashimoto's thyroiditis (35,36). HLA class II molecules have also been reported to be aberrantly expressed on thyroid follicular cells from patients with autoimmune thyroid disease (37,38), but not normal subjects. Hence, we tested the peripheral blood HLA class II in patients with thyroiditis. Although 6 out of 9 patients with thyroiditis shared HLA-DR4-DR53, the sample size is limited to make any conclusive statement about HLA association with this disease.
With this knowledge of ICI-induced thyroiditis and the recent use of these agents in advanced thyroid cancer, the regression of PTC with PD-1 inhibitor therapy for metastatic melanoma in a 40-year-old female prompted us to analyze her immune phenotype and HLA haplotype. Due to tumor regression, enough samples could not be obtained for thyroid flow cytometry but she had similar to more circulating T lymphocytes (CD8+ and CD4+CD8+) and NK cells as compared with ICI-induced thyroiditis and more than healthy volunteers. This patient also shared peripheral blood HLA DR15:01 with 3 PD-1 inhibitor-induced thyroiditis patients. We postulate that thyroid cancer responsiveness is guided by a possible immune similarity to patients who are predisposed to ICI-induced thyroiditis; however, these preliminary observations need to be validated in ICI-treated advanced thyroid cancer patients.
This pilot study has a number of limitations, including the limited sample size leading to lack of power for more comparisons of T cell phenotypes and HLA haplotypes. The absence of a comparison group of ICI-treated patients who did not develop thyroiditis precludes us from reporting with certainty that the immune phenotypes in ICI-induced thyroiditis were not due to the ICI therapy by itself. Healthy thyroid and blood samples were obtained from different groups of volunteers without any thyroid dysfunction or cancer, but those who had thyroid FNA did have benign nonfunctioning thyroid nodules. Lack of flow cytometry in ICI-treated patients before development of thyroiditis precludes us from definitively identifying a predisposing immune phenotype; however, characterizing the immune phenotype at the time of thyroiditis is a significant advance in this field.
To conclude, we have demonstrated that ICI-induced thyroiditis is a T lymphocyte-mediated process with intra-thyroidal predominance of CD8+, PD1+, and CD4−CD8− T lymphocytes. The circulating immune phenotype may also provide a glimpse of the intra-thyroidal status or may suggest additional cell types involved in this process. The HLA haplotype may contribute to this process but requires further evaluation. These findings expand the limited current understanding of ICI-induced thyroiditis, for which identification of an antigen source may translate to novel immunomodulatory therapies for advanced thyroid cancer.
Supplementary Material
Acknowledgment
The authors would like to acknowledge Heather Johnson, LPN who performed blood sample collection for this study.
Disclaimer
The abstract of this work was presented at the 89th Annual Meeting of the American Thyroid Association in Chicago.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH); and Mayo Foundation Small Grants Program.
Supplementary Material
References
- 1. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, Dietz AB, Ryder M. 2017. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab 102:2770–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, Hirota K, Ueda Y, Kanai Y, Yamashita Y, Kondo E, Sone M, Yasoda A, Inagaki N. 2017. Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid 27:894–901 [DOI] [PubMed] [Google Scholar]
- 3. Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. 2014. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 21:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, Merghoub T, Rudin CM, Fish S, Hellmann MD. 2017. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 28:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. 2013. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 98:1361–1375 [DOI] [PubMed] [Google Scholar]
- 6. Torino F, Corsello SM, Salvatori R. 2016. Endocrinological side-effects of immune checkpoint inhibitors. Curr Opin Oncol 28:278–287 [DOI] [PubMed] [Google Scholar]
- 7. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. 2016. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 101:4431–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, Busaidy NL, Subudhi SK, Diab A, Dadu R. 2018. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid 28:1243–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, Onoue T, Takagi H, Hagiwara D, Ito Y, Morishita Y, Gogo M, Suga H, Banno R, Yokota K, Hase T, Morise M, Hashimoto N, Ando M, Kiyoi H, Gotoh M, Ando Y, Akiyama M, Hasegawa Y, Arima H. 2018. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc 2:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gan EH, Mitchell AL, Plummer R, Pearce S, Perros P. 2017. Tremelimumab-induced graves hyperthyroidism. Eur Thyroid J 6:167–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azmat U, Liebner D, Joehlin-Price A, Agrawal A, Nabhan F. 2016. Treatment of ipilimumab induced Graves' disease in a patient with metastatic melanoma. Case Rep Endocrinol 2016:2087525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomer Y. 2010. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid 20:715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobson EM, Huber A, Tomer Y. 2008. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun 30:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nada AM, Hammouda M. 2014. Immunoregulatory T cells, LFA-3 and HLA-DR in autoimmune thyroid diseases. Ind J Endocrinol Metab 18:574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davidson D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jone L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O'Donovan MC, Owen MA, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Breding F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop MG, Connell J, Dominiczak A, Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A; Biologics in RA Genetics and Genomics Study Syndicate (BRAGGS) Steering Committee, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hilder SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S; Breast Cancer Susceptibility Collaboration (UK), Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Newport M, Sirugo G, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Davison D, Ferreira T, Pereira-Gale J, Hallgrimsdo'ttir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Brown MA, Compston A, Farrall M, Hall AS, Hattersley AT, Hill AV, Parkes M, Pembrey M, Stratton MR, Mitchell SL, Newby PR, Brand OJ, Carr-Smith J, Pearce SH, McGinnis R, Keniry A, Deloukas P, Reveille JD, Zhou X, Sims AM, Dowling A, Taylor J, Doan T, Davis JC, Savage L, Ward MM, Learch TL, Weisman MH, Brown M. 2007. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39:1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pardoll DM. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gangadhar TC, Vonderheide RH. 2014. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol 11:91–99 [DOI] [PubMed] [Google Scholar]
- 18. Angell TE, Min L, Wieczorek TJ, Hodi FS. 2018. Unique cytologic features of thyroiditis caused by immune checkpoint inhibitor therapy for malignant melanoma. Genes Dis 5:46–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imblum BA, Baloch ZW, Fraker D, LiVolsi VA. 2019. Pembrolizumab-induced thyroiditis. Endocr Pathol 30:163–167 [DOI] [PubMed] [Google Scholar]
- 20. Kotwal A, Kottschade L, Ryder M. 2020. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid 30:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gustafson MP, Staff NP, Bornschlegl S, Butler GW, Maas ML, Kazamel M, Zubair A, Gastineau DA, Windebank AJ, Dietz AB. 2017. Comprehensive immune profiling reveals substantial immune system alterations in a subset of patients with amyotrophic lateral sclerosis. PLoS One 12:e0182002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, Gastineau DA, Dietz AB. 2015. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PLoS ONE 10:e0121546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gustafson MP, DiCostanzo AC, Wheatley CM, Kim CH, Bornschlegl S, Gastineau DA, Johnson BD, Dietz AB. 2017. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J Immunother Cancer 5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu H, Zheng T, Mao Y, Xu C, Wu F, Bu L, Mou X, Zhou Y, Yuan G, Wang S, Zhou T, Chen D, Mao C. 2016. γδ T cells enhance B cells for antibody production in Hashimoto's thyroiditis, and retinoic acid induces apoptosis of the γδ T cell. Endocrine 51:113–122 [DOI] [PubMed] [Google Scholar]
- 25. Brandt D, Hedrich CM. 2018. TCRalphabeta(+)CD3(+)CD4(-)CD8(-) (double negative) T cells in autoimmunity. Autoimmun Rev 17:422–430 [DOI] [PubMed] [Google Scholar]
- 26. Iwatani Y, Hidaka Y, Matsuzuka F, Kuma K, Amino N. 1993. Intrathyroidal lymphocyte subsets, including unusual CD4+ CD8+ cells and CD3loTCR alpha beta lo/-CD4-CD8- cells, in autoimmune thyroid disease. Clin Exp Immunol 93:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bang K, Lund M, Wu K, Mogensen SC, Thestrup-Pedersen K. 2001. CD4+ CD8+ (thymocyte-like) T lymphocytes present in blood and skin from patients with atopic dermatitis suggest immune dysregulation. Br J Dermatol 144:1140–1147 [DOI] [PubMed] [Google Scholar]
- 28. Chizzolini C, Parel Y, De Luca C, Tyndall A, Akesson A, Scheja A, Dayer JM. 2003. Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor alpha. Arthritis Rheum 48:2593–2604 [DOI] [PubMed] [Google Scholar]
- 29. De Maria A, Malnati M, Moretta A, Pende D, Bottino C, Casorati G, Cottafava F, Melioli G, Mingari MC, Migone N, Romagnani S, Moretta L. 1987. CD3 + 4-8-WT31-(T cell receptor gamma+) cells and other unusual phenotypes are frequently detected among spontaneously interleukin 2-responsive T lymphocytes present in the joint fluid in juvenile rheumatoid arthritis. A clonal analysis. Eur J Immunol 17:1815–1819 [DOI] [PubMed] [Google Scholar]
- 30. Hayday AC. 2000. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 18:975–1026 [DOI] [PubMed] [Google Scholar]
- 31. Chowdhury S, Veyhl J, Jessa F, Polyakova O, Alenzi A, MacMillan C, Ralhan R, Walfish PG. 2016. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 7:32318–32328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lubin D, Baraban E, Lisby A, Jalali-Farahani S, Zhang P, Livolsi V. 2018. Papillary thyroid carcinoma emerging from hashimoto thyroiditis demonstrates increased PD-L1 expression, which persists with metastasis. Endocr Pathol 29:317–323 [DOI] [PubMed] [Google Scholar]
- 33. Farid NR, Stone E, Johnson G. 1980. Graves' disease and HLA: clinical and epidemiologic associations. Clin Endocrinol 13:535–544 [DOI] [PubMed] [Google Scholar]
- 34. Farid NR, Bear JC. 1981. The human major histocompatibility complex and endocrine disease. Endocr Rev 2:50–86 [DOI] [PubMed] [Google Scholar]
- 35. Tandon N, Zhang L, Weetman AP. 1991. HLA associations with Hashimoto's thyroiditis. Clin Endocrinol 34:383–386 [DOI] [PubMed] [Google Scholar]
- 36. Petrone A, Giorgi G, Mesturino CA, Capizzi M, Cascino I, Nistico L, Osborn J, Di Mario U, Buzzetti R. 2001. Association of DRB1*04-DQB1*0301 haplotype and lack of association of two polymorphic sites at CTLA-4 gene with Hashimoto's thyroiditis in an Italian population. Thyroid 11:171–175 [DOI] [PubMed] [Google Scholar]
- 37. Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. 1983. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet 2:1115–1119 [DOI] [PubMed] [Google Scholar]
- 38. Hanafusa T, Pujol-Borrell R, Chiovato L, Russell RC, Doniach D, Bottazzo GF. 1983. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet 2:1111–1115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.