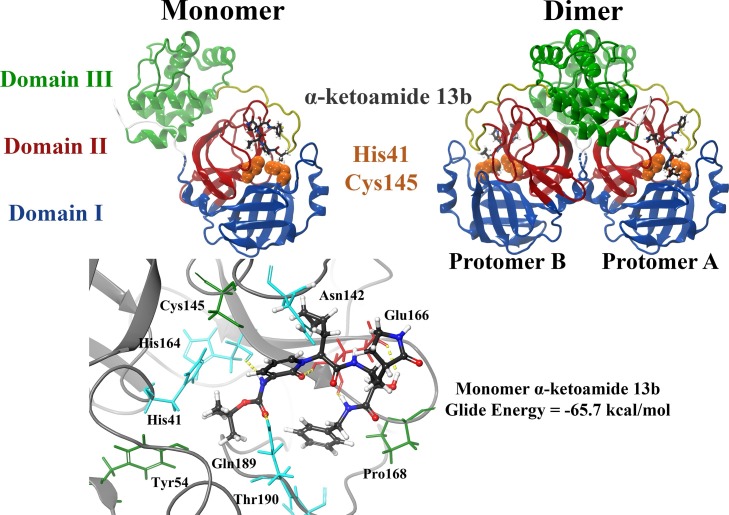
Fig. 1.
Structure of the SARS-CoV-2 Mpro dimer in complex with the α-ketoamide 13b ligand within the substrate-binding site. Mpro consists of three domains, with the catalytic core located between domains I and II. Catalytic dyad residues His41 and Cys145 are highlighted in orange, and the α-ketoamide 13b ligand is shown in grey. The α-ketoamide 13b was docked to the catalytic core using the QPLD protocol of Glide, and interactions with residues are depicted. Hydrogen bonds are shown as dashed yellow lines. Hydrophobic residues are green, polar uncharged residues are cyan, and negatively charged residues are shown in red.